- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Effective Anger Management
By Human Givens College
Have you ever felt powerless in the face of anger? If so, this practical and highly informative course is for you – learn how to take back control… Accredited CPD: 6 hours Distressing, inappropriate and often violent expressions of anger, frustration and rage are on the increase in all areas of public and private life causing mental ill-health, stress, misery and sometimes even physical injury and death – but it doesn’t have to be this way. Learning how to manage anger effectively is key to maintaining a positive mindset and healthy relationships… Having done several courses over the years on handling aggression, I was surprised at how much I could learn from this online anger management courseREGISTERED CARE MANAGER Why take this course The best way to help yourself and others is to understand the causes, triggers and behaviour patterns of anger, and learn the effective techniques for diffusing and coping with aggressive situations. Leading expert Joe Griffin’s online anger management course gives you all of the valuable techniques and the information you need. It covers: anger and health; anger at work; anger in school; road rage; anger and personal safety; anger in relationships; aggressive public out-bursts of rage and much more. It also contains essential information – that will help to keep you safe – if you deal with the general public or work with people suffering from anger disorders. This excellent, well-structured online Anger Management course brings much of what I’m learning into context through practical examples and case histories. Useful in my personal life as well as my working relationshipsLISE BRATTON, EDITOR/ DESIGNER What will you learn Enhanced personal safety in potentially dangerous situations A new ‘toolbox’ of effective anger management strategies for calming down and dealing with angry people Greater understanding about the relationship between anger and emotional and physical health Insight into what triggers your own anger – and how to control it Better communication and conflict resolution skills The Five Myths about anger – that everyone needs to know Information about the effects of anger on our minds and bodies Ways to diffuse potentially violent situations Three invaluable techniques for disarming criticism Why people react aggressively without thinking of the consequences The difference between healthy and destructive anger Understanding road-rage – and how to reduce it The importance of helping children to manage their anger better A better understanding of why anger and aggression are on the increase Ways to inoculate yourself against stress build-up How to deal with passive aggression What makes a ‘rage-aholic’ – why getting angry can become addictive Using non-verbal signals to diffuse situations Improved treatment strategies for helping patients and clients master their uncontrolled anger Course notes Accredited CPD certificate, that also counts towards the Human Givens Diploma and much more… Counts towards a Diploma By taking this course, you could be on your way to completing Part 1 of the Human Givens Diploma – a flexible, part-time psychotherapy course which will deepen your psychological knowledge and increase your ability to help people effectively and quickly. Learn more > Course Programme Part 1Why anger and aggression are on the increase Part 2How to effectively control your own anger Part 3Coping effectively with other people's anger Part 4The aggression inhibition reflex Who is this course suitable for? If you deal professionally with members of the public in difficult, stressful circumstances, this course – presented without jargon – is for you If you counsel or treat angry people, this training is for you If anger is affecting you personally – or you would simply like to deepen your understanding of anger and its effects – this course will be of benefit to you too What's included? 13 Videos 21 Quizzes PDF Course notes Life-long access CPD Certificate Course format The Effective Anger Management online training course is split into 4 modules, each of which are broken down further into different sections containing filmed lectures given in English by the tutor, Joe Griffin. Each film clip is followed by a short series of multiple choice questions. These are designed to help you think about and absorb the course content and to check that you have understood the main points of that section before proceeding to the next. There are also course notes for you to download, plus additional reading information and helpful links. The online course comes with life-long access so you can take as long as you like to work your way through the course and can view it as many times as you like. Once you have completed the online training course, you will receive an accredited CPD certificate, which counts towards Part 1 of the Human Givens Diploma. This course has been independently assessed by the internationally recognised CPD Standards Office for 6 hours of CPD training. Meet your tutor Joe Griffin Joe Griffin is a psychologist with many years’ experience both in psychotherapeutic practice and in training psychotherapists. Read more

How to break the cycle of depression
By Human Givens College
Vital information about depression for everyone – includes new insights and research findings which dramatically improve recovery rates and reduce relapses Accredited CPD: 6 hours Despite being on the increase, depression is actually one of the easiest disorders to treat successfully and quickly – once you know how. The best online depression training I've ever hadMENTAL HEALTH WORKER Why take this course This popular online depression course with psychologist Joe Griffin, a leading expert in the field (and co-founder of the human givens approach), shatters the many myths that still surround this distressing condition and how it should be treated. It gives you the new insights, information and research findings that have been quietly revolutionising the effective treatment of depression for well over a decade. Literally thousands of people in the UK and Ireland have already been helped to recover from depression – often remarkably quickly – as a result of the new information taught on this course. Among other things, you will discover: what really causes depression; why depressed people wake up tired and unmotivated; the link between worrying, dreaming and depression; why some forms of psychotherapy can actually be harmful when treating depression; why the appropriate type of psychotherapy has a dramatically lower rate of relapse than antidepressants and is also the most effective treatment – even with severe cases and postnatal depression. For the sake of the millions of people affected by depression around the world (including rising numbers of children, young people and the elderly), this information needs to be much more widely known. It doesn’t just transform lives – it saves them… So much valuable information about depression – makes total senseGENERAL PRACTITIONER What will you learn Essential new information about why people become depressed A better understanding of what depression really is, why it’s on the increase and how to diagnose it Clear explanations of symptoms such as waking up tired and unmotivated Practical help to quickly break patterns of depression, move people on and prevent relapse New insights into the dissociative elements of depressive lifestyles An important understanding of why some forms of psychotherapy can make depression worse Techniques for tackling rigid thinking and negative expectancy Why some people are more vulnerable to depression than others How to make a positive difference straight away Ways to talk to suicidal people – this training saves lives The myths that have grown up around depression How to manage bi-polar depression psychologically The links between emotional arousal, REM sleep, dreaming and depression How this discovery dramatically improves our ability to help people get out of depression quickly The most effective strategies for successfully treating depression The causes of postnatal depression and how best to treat it How to help with excessive grief Lots of case history examples which illustrate how these new insights combined with the most effective brief therapy techniques can help people recover as quickly as possible Ways to ‘inoculate’ yourself against depression Includes Course Notes Accredited CPD certificate Counts towards a Diploma By taking this course, you could be on your way to completing Part 1 of the Human Givens Diploma – a flexible, part-time psychotherapy course which will deepen your psychological knowledge and increase your ability to help people effectively and quickly. Who is this course suitable for? Anyone wishing to gain a better understanding of the causes and symptoms of depression, and why some people are more vulnerable than others All health and welfare professionals ‒ if you have to deal with, treat or care for depressed people, the new insights on this course will make your work easier and more effective Anyone concerned about the side effects of drug treatments who would like to know about the recent discoveries and easy-to-learn psychotherapeutic techniques that combined can lift depression quickly What's included? 19 Videos 28 Quizzes PDF Course notes Life-long access CPD Certificate Course format ‘How to break the cycle of depression’ is based on our previous one-day seminar of the same name. This online course is split into 4 modules, each of which are broken down further into different sections containing filmed lectures given in English by the tutor, Joe Griffin. Each film clip is followed by a short series of multiple choice questions. These are designed to help you think about and absorb the course content and to check that you have understood the main points of that section before proceeding to the next. There are also course notes for you to download, plus additional reading information and helpful links. You can take as long as you like to work your way through the course and can view it as many times as you like. Once you have completed the online training course, you will receive an accredited CPD certificate, which counts towards Part 1 of the Human Givens Diploma. This course has been independently assessed by the internationally recognised CPD Standards Office for 6 hours of CPD training. Meet your tutor Joe Griffin Joe Griffin is a psychologist with many years’ experience both in psychotherapeutic practice and in training psychotherapists. Read more

Your Head Start in Property
By Property SQ2
Property Investment, learn to get rich slowly, steadily, consistently, by knowing how to find, assess and manage a property investment. Key skills expert property investors use everyday, that make the difference between property headaches and financial freedom through Property Wealth Creation.
Beginners Spanish Course
By iStudy UK
When it comes to speaking Spanish, you need to have a basic knowledge and understanding of the grammar, vocabulary and our Beginners Spanish Course is specifically meant to help you by teaching everything that takes to speak flawlessly in Spanish in a very little time. The course is intended to help you to improve your grasp of Spanish grammar and extend your vocabulary on various topics, such as habits, family, vacation plans, professions, fashion, and much more. You'll learn verbs and how to put your Spanish into practice in daily situations. Upon successful completion of the course, you'll get started speaking in Spanish and can have general conversations in Spanish without making any grammatical mistake. What Will I Learn? Learn Spanish in simple, bite sized chunks. Perfect for the complete beginner with no Spanish knowledge. Be more than just an average tourist when travelling to Spanish speaking countries. Speak from the very first lesson. Build your own sentences without memorisation. Complete lessons in 3 minute chunks - perfect for the busy learner. Learn words and phrases that you can use immediately. Requirements You don't need to know any Spanish to succeed with 3 Minute Spanish. Who is the target audience? You really want to learn to speak Spanish in a very simple and very quick way. You are going to spend a holiday/vacation in Spain or another Spanish speaking country. You have failed to learn Spanish in the past but really want to give it one more go. You have no experience with Spanish but you would like to learn fast. You have always wanted to learn a new language but you are very busy and can never find the time. Introduction Introduction FREE 00:05:00 Lesson: 01 Lesson 1a 00:03:00 Lesson 1b 00:04:00 Lesson 1c 00:04:00 Lesson 1d 00:04:00 Lesson: 02 Lesson 2a 00:03:00 Lesson 2b 00:04:00 Lesson 2c 00:04:00 Lesson 2d 00:04:00 Lesson 2e 00:03:00 Lesson: 03 Lesson 3a 00:04:00 Lesson 3b 00:03:00 Lesson 3c 00:04:00 Lesson 3d 00:03:00 Lesson 3e 00:03:00 Lesson 3f 00:05:00 Lesson: 04 Lesson 4a 00:03:00 Lesson 4b 00:03:00 Lesson 4c 00:03:00 Lesson 4d 00:03:00 Lesson 4e 00:03:00 Lesson 4f 00:03:00 Lesson 4g 00:03:00 Lesson 4h 00:04:00 Lesson: 05 Lesson 5a 00:03:00 Lesson 5b 00:03:00 Lesson 5c 00:03:00 Lesson 5d 00:03:00 Lesson 5e 00:03:00 Lesson 5f 00:03:00 Lesson 5g 00:03:00 Lesson 5h 00:03:00 Lesson 5i 00:03:00 Lesson 5j 00:03:00 Lesson 5k 00:03:00 Lesson: 06 Lesson 6a 00:03:00 Lesson 6b 00:03:00 Lesson 6c 00:03:00 Lesson 6d 00:03:00 Lesson 6e 00:03:00 Lesson 6f 00:03:00 Lesson 6g 00:03:00 Lesson: 07 Lesson 7a 00:03:00 Lesson 7b 00:03:00 Lesson 7c 00:03:00 Lesson 7d 00:03:00 Lesson 7e 00:03:00 Lesson 7f 00:03:00 Lesson 7g 00:03:00 Lesson 7h 00:03:00 Lesson: 08 Lesson 8a 00:03:00 Lesson 8b 00:03:00 Lesson 8c 00:03:00 Lesson 8d 00:04:00 Lesson 8e 00:03:00 Lesson 8f 00:04:00 Lesson 8g 00:03:00 Lesson 8h 00:03:00 Lesson 8i 00:03:00 Lesson 8j 00:03:00 Lesson 8k 00:04:00 Lesson: 09 Lesson 9a 00:03:00 Lesson 9b 00:03:00 Lesson 9c 00:03:00 Lesson 9d 00:03:00 Lesson 9e 00:03:00 Lesson 9f 00:03:00 Lesson 9g 00:03:00 Resources Resources: Beginners Spanish Course 00:00:00 Course Certification

Risk Management & Outsourcing School Excursions, Camps & Activities
By Xcursion
Risk Management & Outsourcing School Excursions, Camps & Activities Do you contract out school camps, excursions or international tours? If so, you need to understand what risks you've transferred & what risks you've retained. This course steps you through exactly what you need to know when contracting something out.

Learn Automation Testing with Java and Selenium Webdriver
By Packt
In this course, you will learn how to write great automation tests with Selenium WebDriver and Java, and start building automation testing frameworks!

VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

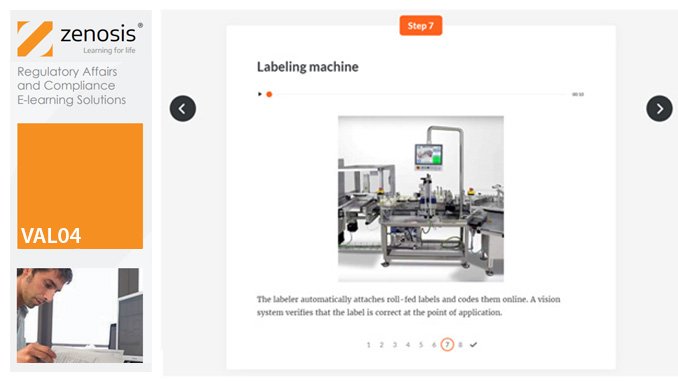
VAL04: Operational and Performance Qualification
By Zenosis
Having undergone Installation Qualification, before equipment can be used routinely in production, it needs to undergo Operational Qualification (OQ) and Performance Qualification (PQ). This module describes OQ and PQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.