- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
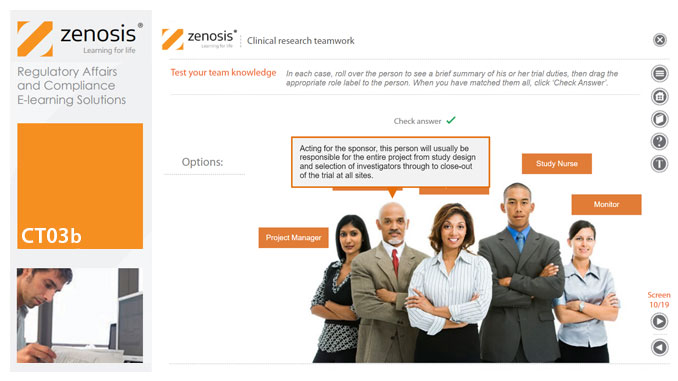
CT03b - Clinical research teamwork
By Zenosis
A clinical trial, particularly a late-phase commercial study, is a major project requiring collaboration between the sponsor and staff or contractor, on the one hand, and the clinical investigator(s) and other healthcare professionals on the other. Good communication among all parties is essential. In this short course we introduce the major roles in a typical clinical research project and outline their duties.
UX Research and Testing for Designers
By Compete High
Introducing 'UX Research and Testing for Designers' Welcome to the ultimate toolkit for designers seeking to elevate their user experience (UX) game! This comprehensive course delves into the intricacies of UX research and testing, equipping designers with indispensable skills to create user-centric designs that truly resonate. Module 1: Introduction to UX Research and Testing Get acquainted with the fundamentals of UX research and testing. Learn why it's crucial, its impact on design decisions, and how it aligns with user needs and business goals. Lay the groundwork for a successful UX journey. Module 2: User Research Methods Explore various user research methodologies to gain valuable insights into user behaviors, preferences, and pain points. From interviews to surveys and ethnographic studies, master the art of extracting actionable data to inform your design decisions. Module 3: Usability Testing Methods Dive deep into usability testing techniques to evaluate the effectiveness and efficiency of your designs. Learn how to conduct tests, gather meaningful feedback, and iterate towards user-friendly interfaces that drive engagement and satisfaction. Module 4: Card Sorting and Information Architecture Uncover the secrets of organizing information effectively with card sorting and information architecture principles. Discover how to structure content intuitively, enhance navigation, and create seamless user experiences that keep users coming back for more. Module 5: User Journey Mapping Embark on a journey mapping expedition to visualize and understand the user experience from end to end. Learn how to identify pain points, moments of delight, and opportunities for optimization, enabling you to craft engaging and cohesive user journeys. Module 6: Remote and Mobile User Testing Navigate the realm of remote and mobile user testing to accommodate today's dynamic digital landscape. Explore innovative tools and methodologies to conduct tests across different devices and environments, ensuring your designs deliver exceptional experiences regardless of platform. Equip yourself with the knowledge and skills needed to revolutionize your design process and create impactful user experiences that stand the test of time. Enroll in 'UX Research and Testing for Designers' today and embark on a transformative journey towards UX excellence. Course Curriculum Module 1_ Introduction to UX Research and Testing Introduction to UX Research and Testing 00:00 Module 2_ User Research Methods User Research Methods 00:00 Module 3_ Usability Testing Methods Usability Testing Methods 00:00 Module 4_ Card Sorting and Information Architecture Card Sorting and Information Architecture 00:00 Module 5_ User Journey Mapping User Journey Mapping 00:00 Module 6_ Remote and Mobile User Testing Remote and Mobile User Testing 00:00

Master the art of strategic business planning with our 'Market Size Mastery' course. Explore market sizing, target audience identification, and competitive analysis. Learn to estimate TAM, SAM, and SOM, and apply market insights for effective business growth. Gain hands-on experience with market sizing tools and resources. Elevate your decision-making with this comprehensive course on market dynamics and successful business strategies.

Professional Certificate in Developing Effective Research Proposal in London 2024
4.9(261)By Metropolitan School of Business & Management UK
The Professional Certificate in Developing Effective Research Proposal aims to introduce the learner to the fundamentals of an effective research proposal by focusing on the details of the content of the research proposal. The course also aims to enable the learner to deal with approval issues of the research proposal. Learning Outcomes After the successful completion of the certificate, the student will be able to learn: The concept of the research proposal. Various outlines of a research report. The concepts of writing up the research. The process of research over time. The sequence of writing up the research. VIDEO - Course Structure and Assessment Guidelines Watch this video to gain further insight. Navigating the MSBM Study Portal Watch this video to gain further insight. Interacting with Lectures/Learning Components Watch this video to gain further insight. Developing Effective Research Proposal The course aims to introduce the learner to the fundamentals of an effective research proposal by focusing on the details of the content of the research proposal. Developing Effective Research Proposal Put your knowledge to the test with this quiz. Read each question carefully and choose the response that you feel is correct. All MSBM courses are accredited by the relevant partners and awarding bodies. Please refer to MSBM accreditation in about us for more details. There are no strict entry requirements for this course. Work experience will be added advantage to understanding the content of the course. The certificate is designed to enhance the learner's knowledge in the field. This certificate is for everyone eager to know more and gets updated on current ideas in their respective field. We recommend this certificate for the following audience. CEO, Director, Manager, Supervisor Research student Business researcher Research supervisor Research Teacher Average Completion Time 2 Weeks Accreditation 1 CPD Hour Level Advanced Start Time Anytime 100% Online Study online with ease. Unlimited Access 24/7 unlimited access with pre-recorded lectures. Low Fees Our fees are low and easy to pay online.

SEO ( Keyword Research), Social Media and Affiliate Marketing - QLS Certificate
By Imperial Academy
3 QLS Endorsed Diploma | QLS Hard Copy Certificate Included | 10 CPD Courses | Lifetime Access | 24/7 Tutor Support
Professional Certificate in Research Data Analysis Methods in London 2024
4.9(261)By Metropolitan School of Business & Management UK
The Professional Certificate in Research Data Analysis Methods aims to provide extensive training on the nature, importance and expected results from qualitative and quantitative data analysis methods so that the learner can decide on the adoption of either or both methods after evaluation of the research objectives. Learning Outcomes After the successful completion of the certificate, the student will be able to learn: Data Analysis. Quantitative Data Analysis. Data Types of Quantitative Data Analysis. Designing Table and charts. Appreciating Data Analytics. Data-Analytic Methods. Analysing Quantitative Data. Qualitative Data analysis. Dimensions of Qualitative Analysis. Qualitative Data Analysis Methods. Analysing Qualitative Data. VIDEO - Course Structure and Assessment Guidelines Watch this video to gain further insight. Navigating the MSBM Study Portal Watch this video to gain further insight. Interacting with Lectures/Learning Components Watch this video to gain further insight. Research Data Analysis Methods The course aims to provide extensive training on the nature, importance and expected results from both qualitative and quantitative data analysis methods. Research Data Analysis Methods Put your knowledge to the test with this quiz. Read each question carefully and choose the response that you feel is correct. All MSBM courses are accredited by the relevant partners and awarding bodies. Please refer to MSBM accreditation in about us for more details. There are no strict entry requirements for this course. Work experience will be added advantage to understanding the content of the course. The certificate is designed to enhance the learner's knowledge in the field. This certificate is for everyone eager to know more and gets updated on current ideas in their respective field. We recommend this certificate for the following audience. CEO, Director, Manager, Supervisor Research student Business researcher Research supervisor Research Teacher Average Completion Time 2 Weeks Accreditation 3 CPD Hours Level Advanced Start Time Anytime 100% Online Study online with ease. Unlimited Access 24/7 unlimited access with pre-recorded lectures. Low Fees Our fees are low and easy to pay online.
Professional Certificate Course in Understanding Academic Research in London 2024
4.9(261)By Metropolitan School of Business & Management UK
Explore the fundamentals of academic research with a focus on source comparison, research processes, and the nuances of primary and secondary research. This course equips participants with the essential skills to navigate the complexities of scholarly inquiry. After the successful completion of the course, you will be able to learn about the following, To analyze the term 'Academic Research and Writing'. To understand different academic writing conventions. To analyze different types of academic research. Throughout the course, participants will first develop a keen understanding of comparing different sources of information and evidence, honing their ability to critically assess data reliability and relevance. Next, they will comprehend the essential steps involved in a research process, acquiring the knowledge needed to navigate from conceptualization to analysis. The course culminates in a thorough exploration of primary and secondary research, providing participants with the expertise to choose and apply these methods judiciously in academic inquiry. This course provides a comprehensive understanding of the principles and practices involved in academic research. Participants will gain proficiency in comparing sources, comprehending the research process, and distinguishing between primary and secondary research. Through a blend of theoretical knowledge and practical application, individuals will be well-equipped to pursue various roles in research and academic settings. Course Structure and Assessment Guidelines Watch this video to gain further insight. Navigating the MSBM Study Portal Watch this video to gain further insight. Interacting with Lectures/Learning Components Watch this video to gain further insight. Understanding how to Conduct Academic Research Self-paced pre-recorded learning content on this topic. Understanding How to Conduct Academic Research Put your knowledge to the test with this quiz. Read each question carefully and choose the response that you feel is correct. All MSBM courses are accredited by the relevant partners and awarding bodies. Please refer to MSBM accreditation in about us for more details. There are no strict entry requirements for this course. Work experience will be added advantage to understanding the content of the course. The certificate is designed to enhance the learner's knowledge in the field. This certificate is for everyone eager to know more and get updated on current ideas in their respective field. We recommend this certificate for the following audience. Research Analyst Academic Writer Research Consultant Content Developer Editorial Assistant Research Coordinator Grant Writer Manuscript Editor Curriculum Developer Publishing Specialist Average Completion Time 2 Weeks Accreditation 3 CPD Hours Level Advanced Start Time Anytime 100% Online Study online with ease. Unlimited Access 24/7 unlimited access with pre-recorded lectures. Low Fees Our fees are low and easy to pay online.

Exploring Focus Groups in Anthropology Research
By The Teachers Training
Exploring Focus Groups in Anthropology Research is yet another 'Teacher's Choice' course from Teachers Training for a complete understanding of the fundamental topics. You are also entitled to exclusive tutor support and a professional CPD-accredited certificate in addition to the special discounted price for a limited time. Just like all our courses, this Exploring Focus Groups in Anthropology Research and its curriculum have also been designed by expert teachers so that teachers of tomorrow can learn from the best and equip themselves with all the necessary skills. Consisting of several modules, the course teaches you everything you need to succeed in this profession. The course can be studied part-time. You can become accredited within 06 Hours studying at your own pace. Your qualification will be recognised and can be checked for validity on our dedicated website. Why Choose Teachers Training Some of our website features are: This is a dedicated website for teaching 24/7 tutor support Interactive Content Affordable price Courses accredited by the UK's top awarding bodies 100% online Flexible deadline Entry Requirements No formal entry requirements. You need to have: Passion for learning A good understanding of the English language Be motivated and hard-working Over the age of 16. Certification CPD Certification from The Teachers Training Successfully completing the MCQ exam of this course qualifies you for a CPD-accredited certificate from The Teachers Training. You will be eligible for both PDF copy and hard copy of the certificate to showcase your achievement however you wish. You can get your digital certificate (PDF) for £4.99 only Hard copy certificates are also available, and you can get one for only £10.99 You can get both PDF and Hard copy certificates for just £12.99! The certificate will add significant weight to your CV and will give you a competitive advantage when applying for jobs. Unit 01: Introduction Welcome to Class! 00:03:00 What is Anthropology? 00:05:00 What is a Focus Group? 00:04:00 The Uses of Focus Groups 00:05:00 Advantages and Disadvantages 00:04:00 Focus Groups in Anthropology 00:05:00 Unit Activity 00:01:00 Unit Activity Answers 00:03:00 Unit 02: Research Ethics Introduction to Research Ethics 00:02:00 History of Research Ethics 00:07:00 Research Ethics 00:06:00 Professional Ethics 00:05:00 Ethics in Focus Group Research 00:09:00 Unit Activity 00:01:00 Unit Activity Answers 00:03:00 Unit 03: Preparing for a Focus Group Deciding on a Research Topic (Part 1) 00:02:00 Deciding on a Research Topic (Part 2) 00:08:00 Deciding on a Research Question 00:08:00 Deciding on a Population and Sample (Part 1) 00:10:00 Deciding on a Population and Sample (Part 2) 00:05:00 Doing a Literature Review 00:14:00 Unit Activity 00:01:00 Activity Answers 00:04:00 Unit 04: Planning for a Focus Group Introduction to Planning for a Focus Group 00:01:00 Overview & Timeline 00:09:00 Budget 00:09:00 Focus Group Setting 00:07:00 Room Arrangement 00:06:00 Unit Activity 00:01:00 Unit Activity Answers 00:03:00 Unit 05: Creating a Focus Group Introduction to Creating a Focus Group 00:01:00 Focus Group Composition 00:10:00 Focus Group Recruiting 00:13:00 Informed Consent 00:08:00 Unit Activity 00:01:00 Unit Activity Answers 00:03:00 Unit 06: Being a Moderator Introduction to Being a Moderator 00:02:00 Duties of a Moderator 00:07:00 Duties of an Assistant 00:06:00 Creating a Discussion Guide 00:08:00 Handling Problems (Part 1) 00:06:00 Handling Problems (Part 2) 00:05:00 Unit Activity 00:01:00 Unit Answers 00:03:00 Unit 07: Conducting a Focus Group Introduction to Conducting a Focus Group 00:01:00 Beginning the Focus Group 00:06:00 Asking Questions & Probes 00:05:00 Focus Group Activities 00:05:00 Taking Field Notes 00:06:00 Ending the Group 00:07:00 Online Focus Groups 00:06:00 Unit Activity 00:01:00 Unit Activity Answers 00:04:00 Unit 08: After a Focus Group Introduction to After a Focus Group 00:02:00 Transcription 00:06:00 Data Analysis (Part 1) 00:15:00 Data Analysis (Part 2) 00:05:00 Sharing Results 00:06:00 Data Management 00:08:00 Unit Activity 00:01:00 Unit Activity Answers 00:04:00 Unit 09: Conclusion Real-World Connections 00:04:00 Activity Answer 00:19:00 Congrats! 00:02:00 BONUS 00:02:00 Resources Resources - Exploring Focus Groups in Anthropology Research 00:00:00


