- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
954 Quality Assurance courses
University First and Second Year - Diploma in Business Management (Level 4&5) - Pathway to BA (Hons) Business Management
4.9(261)By Metropolitan School of Business & Management UK
University First and Second Year - Diploma in Business Management (Level 4&5) - Pathway to BA (Hons) Business Management Level 4 Diploma in Business Management - 8 -12 months - 120 Credits Level 5 Diploma in Business Management - 8 - 12 months - 120 Credits Level 4 is the equivalent of the first year of a Bachelor’s Degree programme. it provides students with 120 university credits upon completion. Level 5 is the equivalent to the second year of a Bachelor’s Degree programme, it provides students with 120 university credits upon completion. It is also equivalent to an HND diploma. Course Details Each module consists of 40 guided learning hours of material with an additional 30-50 hours worth of optional materials which comprise of recommended exercises, recommended readings and internet resources. Within the modules are self-testing exercises. You must take care in answering these. Although they are not marked nor do they count towards your final assessment, the marking is tested against your scoring on the final assessment to check for consistency of score. The modules are written against prescribed learning outcomes defined by the Qualifications and Credit Framework (QCF) administered by Ofqual, the Government appointed regulator. In addition, the learning outcomes are articulated against MSBM Awarding Bodies curriculum who is a recognised awarding body monitored and assessed by QAA, the Quality Assurance Agency, the Government appointed quality assurance body. Successful completion of the full Level 4/5 Extended Diploma in Business Management and final year of an accredited Undergraduate Degree programme, will give students the right credentials to go on and apply for a job in marketing, accounting, human resources, management or business consultancy. Accreditation All MSBM courses are accredited by the relevant partners and awarding bodies. Please refer to MSBM accreditation in about us for more details. University Progression University Top-up On completion of this course, students have the opportunity to complete a Bachelors degree programme from a range of UK universities. The top-up programme can be studied online or on campus. The top-up comprises the final 120 credits which consist of either a dissertation or a dissertation and one module. Examples of University Progression BA (Hons) in Business Management (top-up) awarded by the University of Chichester delivered through London Graduate School. Anglia Ruskin University - BA (Hons) Management (Top-Up). Entry Requirements For entry into Level 4 or 5 Diploma in Business Management, learners must possess the following: * Relevant/Local Board of Examination Result in your Country, which must include at least 5 'C' Grades in English and 4 other subjects. * Mock Examination Result can also be accepted for initial Conditional Admission, pending the release of the Board of Examination Result. (This is assessed on a case by case basis) * Learner must be 18 years or older at the start of the Course. OR * Mature Learners (21 years or older) with work experience in supervisory, leadership or management roles. (This is assessed on a case by case basis). Workshops Workshops are conducted by live webinars for online students. Classroom workshops are available if there is a local branch in your country. Speak our course advisors on this subject. Visa Requirements There is no Visa requirement for this programme.

Moving and Handling of People & Objects Healthcare Train the Trainer
By Passion4Training
The course price includes Refreshments, Car Parking Fees, Workbooks, Resources including access to our Trainer Resource Pack via the Approved Trainer HUB, Approved Trainer Membership, Approved Trainer T-Shirt, Ongoing Quality Assurance , support and Certification. We deliver this course to small groups a maximum of 4 people attend this course at our training premises ensuring all our learners receive Quality time with the Trainers/ Assessors delivering the course.

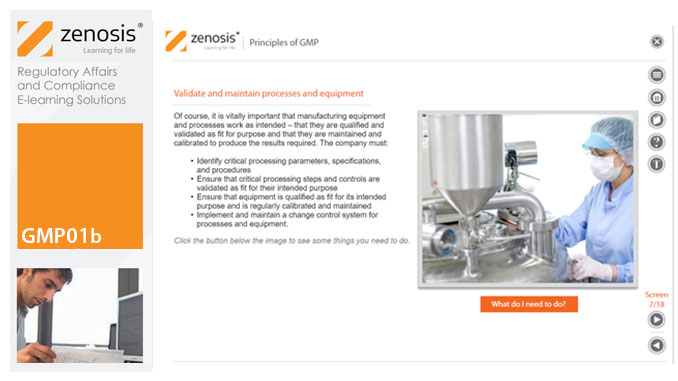
GMP01b - Principles of GMP
By Zenosis
In this short course we present an overview of the main principles of GMP, and we outline some things that manufacturing personnel need to do to comply with requirements. We identify the principal goals of GMP as: prevention of contamination; prevention of mix-ups; scrupulous documentation; validation and maintenance of processes and equipment; quality assurance by an independent unit; and training. We place GMP in the context of a company’s quality management system.
Fast-Moving Consumer Goods (FMCG) & Retail Management - CPD Certified
4.7(47)By Academy for Health and Fitness
Unlock your path to success in the dynamic world of Fast-Moving Consumer Goods (FMCG) & Retail Management with our comprehensive 6-course bundle. As the demand for skilled Retail Managers continues to soar, with job openings growing by 7% annually (source: Bureau of Labor Statistics), our expert-led program equips you with the knowledge and skills to thrive in this booming industry. Don't miss this opportunity to accelerate your career - enrol now and take the first step towards becoming a retail management superstar! This Fast-Moving Consumer Goods (FMCG) & Retail Management Bundle Contains 6 of Our Premium Courses for One Discounted Price: Course 01: Product Management Course 02: Good Manufacturing Practice (GMP) Course 03: Retail Management Course 04: Quality Assurance (QA) Management Course 05: Purchasing and Procurement Course 06: Supply Chain Management Learning Outcomes: Master Product Management strategies for market success. Ensure compliance with Good Manufacturing Practice (GMP) standards. Lead and excel in Retail Management with confidence. Elevate Quality Assurance (QA) Management expertise. Optimise Purchasing and Procurement operations. Streamline Supply Chain Management for efficiency. Gain a holistic understanding of FMCG & Retail dynamics. Embark on a transformative journey through the Fast-Moving Consumer Goods (FMCG) & Retail Management world with our comprehensive 6-course bundle. Each course is carefully designed to empower you with essential skills and insights to thrive in this thriving industry. Product Management: Unlock the secrets of successful product strategies, from ideation to launch, and steer your brand towards market dominance. Good Manufacturing Practice (GMP): Ensure product quality and safety, complying with industry standards, and bolster consumer trust. Retail Management: Acquire the tools to lead, optimise, and innovate in the dynamic realm of retail, ensuring superior customer experiences. Quality Assurance (QA) Management: Become the guardian of product quality, guaranteeing excellence and compliance at every stage. Purchasing and Procurement: Master procurement strategies to cut costs and enhance the efficiency of your supply chain. Supply Chain Management: Streamline operations, minimise costs, and maximise value throughout your supply chain network. CPD 60 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Aspiring Product Managers Quality Control Professionals Retail Enthusiasts Procurement Specialists Requirements You will not need any prior background or expertise. Career path Product Manager: £35,000 - £75,000 Quality Assurance Manager: £30,000 - £65,000 Retail Manager: £25,000 - £60,000 Procurement Specialist: £30,000 - £65,000 Supply Chain Manager: £35,000 - £80,000 FMCG Industry Professional: Salary varies by role and experience. Certificates Digital certificate Digital certificate - Included Hard copy certificate Hard copy certificate - £29 If you are an international student, you will be required to pay an additional fee of 10 GBP for international delivery, and 4.99 GBP for delivery within the UK, for each certificate

Level 3 Assessor course formerly known as A1 TAQA course. Learn More Academy provides all level of Assessor courses in England, Wales and Scotland. This courses was formerly known as TAQA course and this qualification is intended for those who wish to gain an understanding of the principles and practices of assessment without any requirement to practice as assessors. COURSE AIM: This Understanding level consist of: Unit 1: Understanding the principles and practices of assessment D/601/5313 The aim of this unit is to assess the learning and development practitioner’s knowledge and understanding of the principles and practices of assessment. ‘Practitioner’ means anyone with a learning and development responsibility as the whole or a part of their role. Course Outline: • Understanding the legal and good practice in relation to assessment • Developing plans for assessing competence with candidates • Contributing to the internal quality assurance processes • Understanding the principles and practices of assessment • Assessing candidates against agreed learning outcomes • Judging evidence against agreed standards to make assessment decisions • Using a variety of assessment methods • Giving candidates feedback and support on your assessment decisions • Working with other people involved in the assessment process, such as workplace supervisors and other teachers or trainers ENTRY REQUIREMENT: There are no formal requirements for Level 3 Award in Understanding the Principles and Practices of Assessment (TAQA) course, however, candidates should be working in, or working towards a role in quality assurance. COURSE ASSESSMENT: There is no formal examination is required, but at the end of the course you need to submit a portfolio assignment. HOW MUCH THIS COURSE COST? Level 3 Award in Understanding the Principles and Practices of Assessment (TAQA) course will cost for Online Distance Learning £249.99, for Webinar Classroom based course £279.99. There is no any hidden fess/cost.

Level 3 Certificate in Document Control Specialist
By Compliance Central
In the vast expanse of organizational chaos, where files morph into forests and documents roam like wild beasts, there emerges a hero - the Document Control Specialist. Welcome to the Level 3 Certificate in Document Control Specialist, where order reigns supreme and chaos is but a distant memory. Prepare to embark on a journey with Level 3 Certificate in Document Control Specialist course that will transform you into the guardian of order, the sentinel of structure, and the maestro of meticulousness. Step into a world where every document tells a story, and every version carries the weight of history. Through eight meticulously crafted modules, as part of the Level 3 Certificate in Document Control Specialist, we unravel the mysteries of Document Control, from its foundational principles to the intricate strategies that govern its domain. From the lifecycle of documents to the nuances of quality assurance, this Document Control Specialist course is your compass in the tumultuous sea of information management. Become adept at navigating the labyrinthine landscape of project documentation, and master the art of electronic document management systems with ease. With each module of our course, you'll hone your skills, sharpen your instincts, and emerge as a virtuoso in the orchestration of order. So, if you possess a keen eye for detail, a passion for precision, and a penchant for perfection, then join us on this transformative journey with our Document Control Specialist course. With the Level 3 Certificate in Document Control Specialist, you're not just controlling documents - you're sculpting the very fabric of organizational excellence. Learning Outcomes: Understand the principles and elements of Document Control. Navigate the Document Control lifecycle with finesse. Implement effective Document Control strategies and instruments. Manage document quality and ensure adherence to standards. Oversee project document control processes. Master electronic document management systems and soft copy documentation. Level 3 Certificate in Document Control Specialist Module 01: Introduction to Document Control Module 02: Principles of Document Control and Elements of Document Control Environment Module 03: Document Control Lifecycle Module 04: Document Control Strategies and Instruments Module 05: Document Management Module 06: Quality Assurance and Controlling Quality of Documents Module 07: Project Document Control Module 08: Electronic Document Management Systems and Soft Copy Documentation Certificate of Achievement Endorsed Certificate of Achievement from the Quality Licence Scheme Learners will be able to achieve an endorsed certificate after completing the Level 3 Certificate in Document Control Specialist course as proof of their achievement. You can order the endorsed certificate for Free to be delivered to your home by post. For international students, there is an additional postage charge of £10. Endorsement The Quality Licence Scheme (QLS) has endorsed this Level 3 Certificate in Document Control Specialist course for its high-quality, non-regulated provision and training programmes. The QLS is a UK-based organisation that sets standards for non-regulated training and learning. This endorsement means that the Level 3 Certificate in Document Control Specialist course has been reviewed and approved by the QLS and meets the highest quality standards. Who is this course for? Level 3 Certificate in Document Control Specialist Who is this course for? Document Control Specialists Records Managers Compliance Officers Quality Assurance Managers Project Managers Administrative Assistants Requirements Level 3 Certificate in Document Control Specialist To enrol in this Level 3 Certificate in Document Control Specialist course, all you need is a basic understanding of the English Language and an internet connection. Career path Level 3 Certificate in Document Control Specialist Career Paths: Document Control Specialist: £25,000 - £40,000 Records Manager: £30,000 - £45,000 Compliance Officer: £25,000 - £45,000 Quality Assurance Manager: £35,000 - £60,000 Project Manager: £30,000 - £70,000 Certificates CPD Accredited PDF Certificate Digital certificate - Included QLS Endorsed Hard Copy Certificate Hard copy certificate - Included CPD Accredited Hard Copy Certificate Hard copy certificate - £9.99 CPD Accredited Hard Copy Certificate Delivery Charge: Inside the UK: Free Outside of the UK: £9.99 each

Overview Document control policies bring order to the business processes. Therefore, businesses that manage a high volume of data and records establish document control procedures to ensure data is up-to-date and compliant. This Document Control Training will give you a solid introduction to document control processes and practices and discuss how to implement them in your organisation. Training with us, you'll identify the stages of the document lifecycle and enrich your knowledge of document control strategies and instruments. Also, you'll determine the role of document control in quality assurance. The course will also address the different document control requirements for a project and give you clear ideas on electronic document management systems and soft copy documentation. Course Preview Learning Outcomes Realise the importance of document control Discover the procedures involved in document control Learn about the principles of document control and its main elements Determine the relationship between document control and quality assurance Understand the consequences of uncontrolled documents Find expert tips on project document control Learn about electronic document management systems and soft copy documentation. Why Take This Course From John Academy? Affordable, well-structured and high-quality e-learning study materials Meticulously crafted engaging and informative tutorial videos and materials Efficient exam systems for the assessment and instant result Earn UK & internationally recognised accredited qualification Easily access the course content on mobile, tablet, or desktop from anywhere, anytime Excellent career advancement opportunities Get 24/7 student support via email What Skills Will You Learn From the Course? Document Control Quality Assurance Electronic Document Management Who Should Take this Document Control Training? Whether you're an existing practitioner or an aspiring professional, this course is an ideal training opportunity. It will elevate your expertise and boost your CV with key skills and a recognised qualification attesting to your knowledge. Are There Any Entry Requirements? This Document Control Training is available to all learners of all academic backgrounds. But learners should be aged 16 or over to undertake the qualification. And a good understanding of the English language, numeracy, and ICT will be helpful. Document Control Training Certification After completing and passing the Document Control Training successfully, you will be able to obtain a Recognised Certificate of Achievement. Learners can obtain the certificate in hard copy at £14.99 or PDF format at £11.99. Career Pathâ The Document Control Training provides essential skills that will make you more effective in your role. It would be beneficial for any related profession in the industry, such as: Document Controller - Average salary £26,000 per annum Quality Assurance Coordinator - Average salary £27,000 per annum Compliance Officer - Average salary £32,000 per annum Document Control Manager - Average salary £40,000 per annum Module 01: Introduction to Document Control Introduction to Document Control 00:12:00 Module 02: Principles of Document Control and Elements of Document Control Environment Module 02: Principles of Document Control and Elements of Document Control Environment 00:12:00 Module 03: Document Control Lifecycle Document Control Lifecycle 00:11:00 Module 04: Document Control Strategies and Instruments Document Control Strategy and Instruments 00:18:00 Module 05: Document Management Document Management 00:22:00 Module 06: Quality Assurance and Controlling Quality of Documents Quality Assurance and Controlling Quality of Documents 00:11:00 Module 07: Project Document Control Project Document Control 00:12:00 Module 08: Electronic Document Management Systems and Soft Copy Documentation Electronic Document Management System and Soft Copy Documentation 00:18:00 Assignment Assignment - Document Control 00:00:00 Order Your Certificates and Transcript Order Your Certificates and Transcripts 00:00:00

Description Quality Control and Assurance Diploma The Quality Control and Assurance Diploma course is a broad-based programme that provides candidates with both technical and philosophical expertise needed to succeed in the trade. The mass manufacturing of modern-day components and systems established techniques to produce the exact dimensions and layouts. However, these mechanisms were not universal, and as a result, they led to dissatisfaction among the consumers. Quality control indicates the steps undertaken to check for defects in a product, as opposed to the decision to launch an item or service, which may or may not be influenced by financial constraints. The inability to control quality is one of the most important reasons for terminating contracts, especially when the contractors are government and public agencies. Anvils, for example, did not have holes and would not have been meant as removable components. Creating a diagram of the ideal product or service may be one of the most fundamental parts of Quality Control. The concept of releasing a product or service with a design that does not match the product or service will be discarded in a conventional launch process. Over time, however, manufacturers discovered that producing parts that matched their illustrations were rather complex and costly. Consequently, tolerance limitations were established about 1840, stating that a product would work if its components met tolerances. Plug gauges and ring gauges were used to ensure product quality. Nevertheless, this was not a practical solution since discarding or recycling the waste and reducing the number of defects increased production costs. In reality, many approaches have been suggested to prioritise quality control problems and determine whether to ignore them or apply quality assurance measures to optimise and stabilise operations. Quality Control and Assurance do more than save money; they significantly impact a company's long-term viability. Moreover, Quality Control and Assurance are essential tools that let organisations achieve client and consumer trust while enhancing their competitive edge. This has resulted in Quality Control and Assurance becoming a necessary piece of almost every organisation. The Quality Control and Quality Assurance function assists companies in providing high-quality products and services. The Quality Control and Assurance Diploma course provides QA knowledge such that business losses are reduced while playing an essential part in the company's uniqueness and competitiveness. A necessary function of Quality Control and Quality Assurance is to ensure that all procedures run smoothly and efficiently. Quality Control and Assurance Diploma will teach individuals to understand the standards for quality control of products and services, ensuring that the product or service has the features that the client requested and preventing the consumer from acquiring a product with defects or errors. The Quality Control and Assurance Diploma courses will teach people how to deliver the required services or products on time and within budget. The Quality Control and Assurance Diploma provides an overview of all the core elements of quality assurance, an easy way to avoid spending time and energy looking for additional courses to develop your knowledge further. Communication Skills, Purchasing and Procurement, Compliance Manager, Logistics and Operations Management and Lean Six Sigma Certification are covered in the Quality Control and Assurance Diploma course. What you will learn 1: Introduction to Quality Control 2: Improving results with Quality Control 3: Using Quality Control Fundamentals 4: Measuring Quality Process 5: Lean Processes 6: Value Stream Mapping 7: 5S method 8: Six Sigma 9: Theory of Constraints 10: Incorporating quality into processes or products Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.

ISO 9001 Lead Auditor
By Training Centre
The ISO 9001 Lead Auditor training enables you to develop the necessary expertise to perform a Quality Management System (QMS) audit by applying widely recognized audit principles, procedures and techniques. About This Course During this training course, you will acquire the knowledge and skills to plan and carry out internal and external audits in compliance with ISO 19011 and the certification process according to ISO/IEC 17021-1. Based on practical exercises, you will be able to master the audit techniques and become competent to manage an audit program, audit team, communication with customers, and conflict resolution. After acquiring the necessary expertise to perform this audit, you can sit for the exam and gain "Certified ISO 9001 Lead Auditor' credential. By holding this Certificate,, you will demonstrate that you have the capabilities and competencies to audit organizations based on best practices. Learning objectives Understand the operations of a Quality Management System (QMS) based on ISO 9001 Acknowledge the correlation between ISO 9001 and other standards and regulatory frameworks Understand the auditor's role to: plan, lead and follow-up on a management system audit in accordance with ISO 19011 Learn how to lead an audit and audit team Learn how to interpret the requirements of ISO 9001 in the context of a QMS audit Acquire the competencies of an auditor to: plan an audit, lead an audit, draft reports, and follow-up on an audit in compliance with ISO 19011 Educational approach This training is based on both theory and best practices used in QMS audits Lecture sessions are illustrated with practical questions and examples Practical exercises include examples and case study discussions Practice tests are similar to the Certification Exam Prerequisites A fundamental understanding of ISO 9001 and comprehensive knowledge of audit principles. What's Included? Refreshments & Lunch (Classroom only) Course Slide Deck Official Study Guides The Exam Who Should Attend? Auditors seeking to perform and lead Quality Management System (QMS) certification audits Managers or consultants seeking to master a Quality Management System audit process Individuals responsible for maintaining conformance with QMS requirements Technical experts seeking to prepare for a Quality Management System audit Expert advisors in Quality Management Accreditation Our Guarantee We are an approved IECB Training Partner. You can learn wherever and whenever you want with our robust classroom and interactive online training courses. Our courses are taught by qualified practitioners with commercial experience. We strive to give our delegates the hands-on experience. Our courses are all-inclusive with no hidden extras. The one-off cost covers the training, all course materials, and exam voucher. Our aim: To achieve a 100% first time pass rate on all our instructor-led courses. Our Promise: Pass first time or 'train' again for FREE. *FREE training and exam retake offered Assessment Delegates sit a combined exam, consisting of in-course quizzes and exercises, as well as a final 12 question, essay type exam on Day 4 of the course. The overall passing score is 70%, to be achieved within the 150 minute time allowance. Exam results are provided within 24 hours, with both a Certificate and a digital badge provided as proof of success. Provided by This course is Accredited by NACS and Administered by theIECB

ISO 9001 Lead Implementer
By Training Centre
This training course aims to equip you with in-depth knowledge on ISO 9001 requirements, as well as the best practices and approaches used for the implementation and subsequent maintenance of a QMS. By attending this training course, you can help organizations utilize a structured and evidence-based approach for managing the quality of their products and services. Apart from this, you will also learn about the importance of customer focus and benefits of setting the foundations of an organizational culture which enables and supports quality. The training course is followed by a certification exam. If you pass, you can gain the "Certified ISO 9001 Lead Implementer' credential. This certificate validates your competence to implement a QMS based on the requirements of ISO 9001. About This Course Learning objectives By the end of this training course, the participant will be able to: Explain the fundamental concepts and principles of a quality management system (QMS) based on ISO 9001 Interpret the requirements of ISO 9001 for a QMS from the perspective of an implementer Initiate and plan the implementation of a QMS based on ISO 9001, by utilizing best practice Support an organization in operating, maintaining, and continually improving a QMS based on ISO 9001 Prepare an organization to undergo a third-party certification audit Educational approach This training course is learner-centred and contains: Theories, approaches, and best practices used in management system implementation, operation, maintenance, and continual improvement Theoretical basis supported by practical examples, throughout the four days of the training course Interaction between the trainers and participants by means of questions and discussions Essay-type homework exercises at the end of each day Quizzes with stand-alone items (after each section) and scenario-based quizzes (at the end of each day), intended to prepare the participants for the certification exam Accreditation Assessment The exam covers the following competency domains: Domain 1: Fundamental principles and concepts of a quality management system Domain 2: Initiation of a QMS implementation Domain 3: Planning of a QMS implementation based on ISO 9001 Domain 4: Implementation of a QMS based on ISO 9001 Domain 5: Monitoring and measurement of a QMS based on ISO 9001 Domain 6: Continual improvement of a QMS based on ISO 9001 Domain 7: Preparation for a QMS certification audit The exam itself is a 12 question, essay type format, to be completed within a 150 minute window. The exam pass mark is 70%. Exam results are provided within 24 hours. Our Guarantee We are an official IECB Training Provider If you fail an exam, you can try again for free If you feel you need additional training, you can train for free too Prerequisites The main requirements for participating in this training course are a basic knowledge of ISO management system standards, overall knowledge of ISO 9001, and the MS implementation principles. An understanding of ISO's quality management principles may also facilitate the learning process. What's Included? Official Study Guides 4 day's Instructor led training Exam fees Who Should Attend? The ISO 9001 Lead Implementer training course is intended for: Personnel responsible for maintaining and improving the quality of the products and services of the organization Personnel responsible for meeting customer requirements Consultants, advisors, professionals wishing to obtain in-depth knowledge of ISO 9001 requirements for a QMS Professionals wishing to acquaint themselves with best practice methodology for implementing a QMS Individuals responsible for maintaining the conformity of QMS to ISO 9001 requirements Members of QMS implementation and operation teams Individuals aspiring to pursue a career in quality management Provided by This course is Accredited by NACSand Administered by the IECB.

Search By Location
- Quality Assurance Courses in London
- Quality Assurance Courses in Birmingham
- Quality Assurance Courses in Glasgow
- Quality Assurance Courses in Liverpool
- Quality Assurance Courses in Bristol
- Quality Assurance Courses in Manchester
- Quality Assurance Courses in Sheffield
- Quality Assurance Courses in Leeds
- Quality Assurance Courses in Edinburgh
- Quality Assurance Courses in Leicester
- Quality Assurance Courses in Coventry
- Quality Assurance Courses in Bradford
- Quality Assurance Courses in Cardiff
- Quality Assurance Courses in Belfast
- Quality Assurance Courses in Nottingham