- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
11862 Professional Development courses delivered Online
Google Ads and Digital Marketing Courses Online Training Bundle
By Study Plex
Recognised Accreditation This course is accredited by continuing professional development (CPD). CPD UK is globally recognised by employers, professional organisations, and academic institutions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. The Quality Licence Scheme is a brand of the Skills and Education Group, a leading national awarding organisation for providing high-quality vocational qualifications across a wide range of industries. What is CPD? Employers, professional organisations, and academic institutions all recognise CPD, therefore a credential from CPD Certification Service adds value to your professional goals and achievements. Benefits of CPD Improve your employment prospects Boost your job satisfaction Promotes career advancement Enhances your CV Provides you with a competitive edge in the job market Demonstrate your dedication Showcases your professional capabilities What is IPHM? The IPHM is an Accreditation Board that provides Training Providers with international and global accreditation. The Practitioners of Holistic Medicine (IPHM) accreditation is a guarantee of quality and skill. Benefits of IPHM It will help you establish a positive reputation in your chosen field You can join a network and community of successful therapists that are dedicated to providing excellent care to their client You can flaunt this accreditation in your CV It is a worldwide recognised accreditation What is Quality Licence Scheme? This course is endorsed by the Quality Licence Scheme for its high-quality, non-regulated provision and training programmes. The Quality Licence Scheme is a brand of the Skills and Education Group, a leading national awarding organisation for providing high-quality vocational qualifications across a wide range of industries. Benefits of Quality License Scheme Certificate is valuable Provides a competitive edge in your career It will make your CV stand out Course Curriculum Digital Marketing Google Ads Masterclass Search Engine Optimization Training Advanced SEO Training Master In SEO Keyword Research Instagram Marketing Course Affiliate Marketing Social Media Influencer Digital Advertising and Marketing 101: The Complete Guide Digital Advertising and Marketing 201: Top Topic & Trends Digital Advertising and Marketing 301: Professional Course Digital Marketing Email Marketing Masterclass 2 Master In Landing Page Design Obtain Your Certificate Order Your Certificate of Achievement 00:00:00 Email Marketing Masterclass

Executive Coaching Certification - Everything you wanted to know about it
By Study Plex
Recognised Accreditation This course is accredited by continuing professional development (CPD). CPD UK is globally recognised by employers, professional organisations, and academic institutions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. The Quality Licence Scheme is a brand of the Skills and Education Group, a leading national awarding organisation for providing high-quality vocational qualifications across a wide range of industries. What is CPD? Employers, professional organisations, and academic institutions all recognise CPD, therefore a credential from CPD Certification Service adds value to your professional goals and achievements. Benefits of CPD Improve your employment prospects Boost your job satisfaction Promotes career advancement Enhances your CV Provides you with a competitive edge in the job market Demonstrate your dedication Showcases your professional capabilities What is IPHM? The IPHM is an Accreditation Board that provides Training Providers with international and global accreditation. The Practitioners of Holistic Medicine (IPHM) accreditation is a guarantee of quality and skill. Benefits of IPHM It will help you establish a positive reputation in your chosen field You can join a network and community of successful therapists that are dedicated to providing excellent care to their client You can flaunt this accreditation in your CV It is a worldwide recognised accreditation What is Quality Licence Scheme? This course is endorsed by the Quality Licence Scheme for its high-quality, non-regulated provision and training programmes. The Quality Licence Scheme is a brand of the Skills and Education Group, a leading national awarding organisation for providing high-quality vocational qualifications across a wide range of industries. Benefits of Quality License Scheme Certificate is valuable Provides a competitive edge in your career It will make your CV stand out Course Curriculum Introduction Introduction 00:03:00 What is Executive Coaching aka Leadership Coaching 00:02:00 Why is Executive Coaching so Popular Why is Executive Coaching so Popular Introduction 00:01:00 The Fundamental Problem With Most Leadership Development Methods 00:03:00 Leadership Development is Very Similar to Achieving Fitness Goals. 00:04:00 Executive Coaching Process is Both Time and Resource Efficient 00:01:00 Confidentiality And a âSafeâ Environment - to The Leader 00:03:00 The Mid-career Crisis and How To Overcome it Intro to this Section 00:01:00 Hitting The Plateau 00:02:00 The Traditional View of Professional Development 00:01:00 A Coaching Approach to Professional Development 00:06:00 What is The Difference Between Executive coaching and Leadership Coaching Executive coaching Vs Leadership Coaching 00:05:00 Overview of The Executive Coaching Process Introduction 00:01:00 Leadership Development Goals 00:01:00 Knowing Your Strengths and Improvement Areas 00:06:00 What is 360-Degree Feedback 00:04:00 Deciding on Improvement Areas 00:02:00 Hiring a Leadership Coach to Debrief 00:01:00 What Happens in An Executive Coaching Session? What Happens in An Executive Coaching Session 00:06:00 What happens IN BETWEEN the coaching sessions? What happens IN BETWEEN the Coaching Sessions 00:03:00 How To Select The Right Executive Coach For You Introduction 00:02:00 Supportive and Challenge 00:03:00 Honest Feedback & Structured Process 00:04:00 Confidentiality and Altering Perception 00:03:00 Role Model of Behaviour 00:02:00 Qualification & Experience 00:03:00 Certificate of Achievement Certificate of Achievement 00:00:00 Get Your Insurance Now Get Your Insurance Now 00:00:00 Feedback Feedback 00:00:00

24 Hour Flash Deal **33-in-1 Payroll Officer Mega Bundle** Payroll Officer Enrolment Gifts **FREE PDF Certificate**FREE PDF Transcript ** FREE Exam** FREE Student ID ** Lifetime Access **FREE Enrolment Letter ** Take the initial steps toward a successful long-term career by studying the Payroll Officer package online with Studyhub through our online learning platform. The Payroll Officer bundle can help you improve your CV, wow potential employers, and differentiate yourself from the mass. This Payroll Officer course provides complete 360-degree training on Payroll Officer. You'll get not one, not two, not three, but 33 Payroll Officer courses included in this course. Plus Studyhub's signature Forever Access is given as always, meaning these Payroll Officer courses are yours for as long as you want them once you enrol in this course. This Payroll Officer Bundle consists the following career oriented courses: Diploma in Payroll (UK Payroll System, Payee, Tax, NI, Pension) at QLS Level 5 Payroll Administrator Diploma in Accountancy Cashflow Management and Forecasting Financial Planning Corporate Risk And Crisis Management Financial Analysis Methods Finance: Financial Risk Management Corporate Finance: Working Capital Management Capital Budgeting & Investment Decision Rules Fundamentals of Corporate Finance Financial Modelling Financial Modeling Course for a Non-Finance Background Sage 50 Accounts and Projects Creation Training Actuary Certification: Navigating the Complex World of Risk and Finance Anti-Money Laundering (AML) Know Your Customer (KYC) Financial Crime Officer Data Protection and Data Security (GDPR) Training Certification Navigating Sanctions Risk: A Comprehensive Management Guide International Banking: Global Operations and Cross-Border Transactions Financial Management Professional Training Certification Risk Assessment & Management Personal Credit Control Business Finance Time Management Creative Thinking: Techniques and Tools for Problem Solving Corporate Relationship Goals for Success Assertiveness Skills: Developing Confidence and Effective Communication Emotional Intelligence and Human Behaviour Interpersonal Skills for Professional Development: Building Effective Relationships Business Networking Skills Decision Making and Critical Thinking Online Course In this exclusive Payroll Officer bundle, you really hit the jackpot. Here's what you get: Step by step Payroll Officer lessons One to one assistance from Payroll Officerprofessionals if you need it Innovative exams to test your knowledge after the Payroll Officercourse 24/7 customer support should you encounter any hiccups Top-class learning portal Unlimited lifetime access to all 33 Payroll Officer courses Digital Certificate, Transcript and student ID are all included in the price PDF certificate immediately after passing Original copies of your Payroll Officer certificate and transcript on the next working day Easily learn the Payroll Officer skills and knowledge you want from the comfort of your home The Payroll Officer course has been prepared by focusing largely on Payroll Officer career readiness. It has been designed by our Payroll Officer specialists in a manner that you will be likely to find yourself head and shoulders above the others. For better learning, one to one assistance will also be provided if it's required by any learners. The Payroll Officer Bundle is one of the most prestigious training offered at StudyHub and is highly valued by employers for good reason. This Payroll Officer bundle course has been created with 33 premium courses to provide our learners with the best learning experience possible to increase their understanding of their chosen field. This Payroll Officer Course, like every one of Study Hub's courses, is meticulously developed and well researched. Every one of the topics is divided into Payroll Officer Elementary modules, allowing our students to grasp each lesson quickly. The Payroll Officer course is self-paced and can be taken from the comfort of your home, office, or on the go! With our Student ID card you will get discounts on things like music, food, travel and clothes etc. CPD 330 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Payroll Officer training is suitable for - Students Recent graduates Job Seekers Individuals who are already employed in the relevant sectors and wish to enhance their knowledge and expertise in Payroll Officer. Requirements To participate in this Payroll Officer course, all you need is - A smart device A secure internet connection And a keen interest in Payroll Officer. Career path You will be able to kickstart your Payroll Officer career because this course includes various courses as a bonus. This Payroll Officeris an excellent opportunity for you to learn multiple skills from the convenience of your own home and explore Payroll Officer career opportunities. Certificates CPD Accredited Certificate Digital certificate - Included CPD Accredited Hardcopy Certificate - Free Enrolment Letter - Free Student ID Card - Free Please Note: Studyhub is a Compliance Central approved resale partner for Quality Licence Scheme Endorsed courses.

24 Hour Flash Deal **33-in-1 Training Manager Mega Bundle** Training Manager Enrolment Gifts **FREE PDF Certificate**FREE PDF Transcript ** FREE Exam** FREE Student ID ** Lifetime Access **FREE Enrolment Letter ** Take the initial steps toward a successful long-term career by studying the Training Manager package online with Studyhub through our online learning platform. The Training Manager bundle can help you improve your CV, wow potential employers, and differentiate yourself from the mass. This Training Manager course provides complete 360-degree training on Training Manager. You'll get not one, not two, not three, but 33 Training Manager courses included in this course. Plus Studyhub's signature Forever Access is given as always, meaning these Training Manager courses are yours for as long as you want them once you enrol in this course. This Training Manager Bundle consists the following career oriented courses: Employee Training Responsibilities: Effective Training Management Train the Trainer Certificate Conflict Resolution Workplace Communication Management Influencing and Negotiation Skills Diversity Training Performance Management Diploma in Workplace Professionalism and Communication Motivational Mentorship: Inspiring Personal and Professional Growth Mastering Organizational Chaos Organizational Behaviour, HR and Leadership Employee Engagement and Retention Strategies Accredited Mediator Skills Training: Mediation & Dispute Resolution Corporate Relationship Goals for Success Team Building Masterclass Strategic Management and Leadership Development Change Management UK Employment Law Workplace Health and Safety Advanced Diploma Motivating, Performance Managing and Maintaining Team Culture in a Remote Team Workplace Health and Safety Advanced Diploma Cross-Cultural Awareness Training Diploma Certificate Supporting a Grieving Employee: A Manager's Guide Employee Hiring and Termination Training Safety Awareness 101 for New Employee Time Management Creative Thinking: Techniques and Tools for Problem Solving Corporate Relationship Goals for Success Assertiveness Skills: Developing Confidence and Effective Communication Emotional Intelligence and Human Behaviour Interpersonal Skills for Professional Development: Building Effective Relationships Business Networking Skills Decision Making and Critical Thinking Online Course In this exclusive Training Manager bundle, you really hit the jackpot. Here's what you get: Step by step Training Manager lessons One to one assistance from Training Managerprofessionals if you need it Innovative exams to test your knowledge after the Training Managercourse 24/7 customer support should you encounter any hiccups Top-class learning portal Unlimited lifetime access to all 33 Training Manager courses Digital Certificate, Transcript and student ID are all included in the price PDF certificate immediately after passing Original copies of your Training Manager certificate and transcript on the next working day Easily learn the Training Manager skills and knowledge you want from the comfort of your home The Training Manager course has been prepared by focusing largely on Training Manager career readiness. It has been designed by our Training Manager specialists in a manner that you will be likely to find yourself head and shoulders above the others. For better learning, one to one assistance will also be provided if it's required by any learners. The Training Manager Bundle is one of the most prestigious training offered at StudyHub and is highly valued by employers for good reason. This Training Manager bundle course has been created with 33 premium courses to provide our learners with the best learning experience possible to increase their understanding of their chosen field. This Training Manager Course, like every one of Study Hub's courses, is meticulously developed and well researched. Every one of the topics is divided into Training Manager Elementary modules, allowing our students to grasp each lesson quickly. The Training Manager course is self-paced and can be taken from the comfort of your home, office, or on the go! With our Student ID card you will get discounts on things like music, food, travel and clothes etc. CPD 330 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Training Manager training is suitable for - Students Recent graduates Job Seekers Individuals who are already employed in the relevant sectors and wish to enhance their knowledge and expertise in Training Manager. Requirements To participate in this Training Manager course, all you need is - A smart device A secure internet connection And a keen interest in Training Manager. Career path You will be able to kickstart your Training Manager career because this course includes various courses as a bonus. This Training Manageris an excellent opportunity for you to learn multiple skills from the convenience of your own home and explore Training Manager career opportunities. Certificates Training Manager Digital certificate - Included CPD Accredited Hardcopy Certificate - Free Enrolment Letter - Free Student ID Card - Free Please Note: Studyhub is a Compliance Central approved resale partner for Quality Licence Scheme Endorsed courses.

Join our Women in Insurance Leadership Workshop and gain insights from industry experts on how to succeed in the male-dominated insurance sector. This workshop is designed to empower women by providing valuable tools and resources to enhance leadership skills, build professional networks, and create a more inclusive workplace culture. Don't miss this opportunity to connect with other women in the industry and take your career to the next level. Register today!

Report Writing
By Step Into Learning
A report is a short, sharp, concise document which is written for a particular purpose and audience. It generally sets outs and analyses a situation or problem, often making recommendations for future action. It is a factual paper, and needs to be clear and well-structured. This course will help you hone your report writing skills

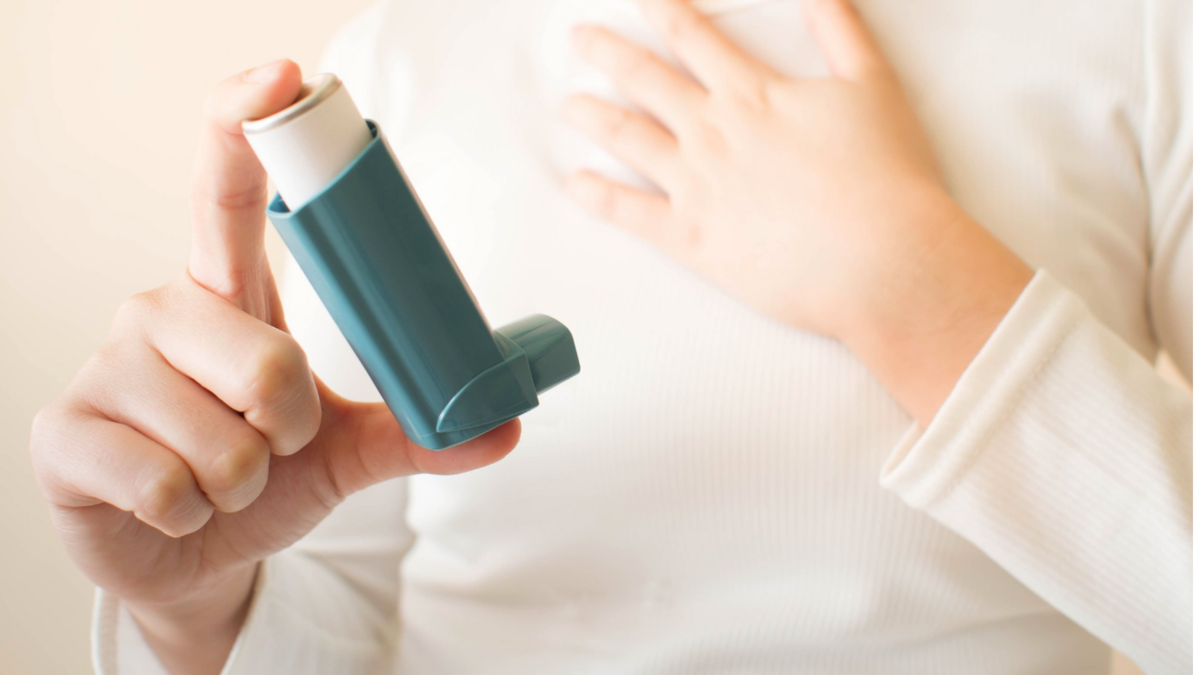
Asthma Update
By BBO Training
This comprehensive one-day course is designed to empower registered healthcare professionals (HCPs) practicing in primary care with the latest advancements and insights in the field of asthma care. Building on the participants' existing expertise, this update day aims to enhance their proficiency in managing asthma effectively and providing the highest level of patient care. Target Audience: Exclusively tailored for registered healthcare professionals already experienced in asthma care within the primary care setting, this course is a unique opportunity for these practitioners to stay current with the latest developments in the field. Course Benefits: This Asthma Update Day is a prime opportunity for seasoned primary care healthcare professionals to deepen their expertise in asthma care. By staying current with the latest developments, practitioners can enhance their ability to provide high-quality, up-to-date patient care. The course encourages interactive learning, networking, and problem-solving, fostering a dynamic and collaborative learning environment. Prerequisites: This course is exclusively designed for registered healthcare professionals already actively practicing in the field of asthma care within the primary care setting. Outcome: Participants will leave the course armed with new insights, strategies, and approaches to further elevate their asthma care practice, ultimately improving patient outcomes and contributing to the advancement of respiratory healthcare within primary care. Asthma Update Day - Learning Objectives · Understanding Asthma Diagnosis Essentials o Gain insight into the latest diagnostic criteria and techniques for asthma. o Discuss the challenges and methods in accurately diagnosing asthma in children. · Investigative Tests and Guideline Exploration o Review the range of investigative tests available for diagnosing and managing asthma. o Examine the current BTS/NICE & GINA Guidelines and their application in clinical practice. · Pharmacological Treatments and Inhaler Techniques o Update knowledge on the pharmacology of asthma treatments. o Develop practical skills in various inhaler techniques and understand their importance in patient care. · Personalised Asthma Management o Understand the factors influencing the choice of inhalers. o Learn to develop and implement Personalised Asthma Action Plans (PAAPs). o Explore SMART/MART therapy and its role in managing asthma. · Managing Asthma Exacerbations o Identify and manage asthma exacerbations effectively. o Discuss strategies for preventing and managing severe asthma episodes. · Application Through Case Studies o Engage in interactive case studies to apply learning in real-world scenarios. o Encourage the sharing of experiences and best practices among participants. · Conclusion and Future Directions o Summarise key takeaways from the day's sessions. o Discuss future trends and areas for continued professional development in asthma care. Through the "Asthma Update Day" course, clinicians will leave with an elevated level of confidence and competence, empowered to provide enhanced asthma care and positively influence patient outcomes within the primary care setting.
Level 3 Award - Supporting Teaching and Learning
By Kingston Open College
NCFE Awarded Ultimate Teaching Assistant Qualification Pack - In Buy1 Get 9 Roaring Offer | No Hidden FEE

This Learning Support Assistant bundle offers a thorough introduction to key roles within educational support, ideal for those looking to enhance their knowledge and confidence in school settings. The courses cover essential areas including supporting learners, understanding the coordination of special educational needs, and foundational early years teaching assistant skills. Designed to build a solid theoretical foundation, this bundle is suitable for anyone aiming to better understand the educational environment without the need for in-person attendance. Each course is carefully structured to provide clear insights into the responsibilities and duties involved in supporting children and young people’s learning and development. The SENCO module delves into the strategic role of managing special educational needs, while the EYFS course focuses on early years frameworks and learning support. This bundle ensures a balanced understanding across various educational support roles, making it an excellent resource for those pursuing a career in education or seeking to expand their professional skill set in a flexible, accessible format. These comprehensive courses are available in this Learning Support Assistant - CPD Accredited 3 Courses Bundle Course 01: Learning Support Assistant Course 02: SENCO - Special Educational Needs Coordination Course 03: EYFS Teaching Assistant ** Special Offer: Free PDF and Hard Copy Certificates** Key Benefits Get instant PDF and Hard Copy certificate Fully online courses Developed by professionals Self-paced learning and laptop, tablet, and smartphone-friendly 24/7 Learning Assistance Course Curriculum: Module-01: The Roles of a Learning Support Assistant (LSA) Module-02: Working with Children in a School Module-03: Assessing Students Module-04: Skills of a Learning Support Assistant Module-05: Providing for All Children Module-06: Safeguarding and Wellbeing of Children and Young People Module-07: Dealing with Emergencies Module-08: Special Educational Needs (SEN) Module-09: Professional Development Module-10: Time and Stress Management Course Assessment You will immediately be given access to a specifically crafted MCQ test upon completing each Learning Support Assistant - CPD Accredited 3 Courses Bundle bundle course. For each test, the pass mark will be set to 60%. Accredited Certificate After successfully completing this Learning Support Assistant - CPD Accredited 3 Courses Bundle course, you will qualify for the CPD Quality Standards (CPD QS) certified certificate from Training Express. CPD 30 CPD hours / points Accredited by The CPD Quality Standards (CPD QS) Who is this course for? The Learning Support Assistant - CPD Accredited 3 Courses Bundle training is ideal for highly motivated individuals or teams who want to enhance their skills and efficiently skilled employees. Requirements There are no formal entry requirements for the course, with enrollment open to anyone! Career path Learn the essential skills and knowledge you need to excel in your professional life with the help & guidance from our Learning Support Assistant - CPD Accredited 3 Courses Bundle training.

Introducing the ultimate knowledge powerhouse for aspiring surveying professionals - the Surveying (Building, Land and Development) QLS Endorsed bundle! Unlock the doors to a thriving career in surveying with this comprehensive collection of eight meticulously curated courses. Whether you're a seasoned practitioner or just starting your journey, this bundle is your passport to success. Within this bundle, you'll find three QLS-endorsed courses that carry the weight of professional recognition. These courses have been rigorously evaluated and approved by the prestigious QLS, ensuring the highest standards of quality and excellence. And that's not all - upon completion of these endorsed courses, you'll receive exclusive hardcopy certificates, a tangible symbol of your mastery in building quantity surveying, land surveying, and property development. Let your achievements speak volumes as you proudly display these certificates, highlighting your commitment to excellence. But we didn't stop there. We understand the need for continuous professional development, which is why we've included five CPD QS-accredited courses in this bundle. Expand your expertise with topics like construction estimation using RSMeans, LEED V4 - Building Design and Construction, WELL Building Standard, Construction Industry Scheme (CIS), and Bricklaying and Construction. These courses, meticulously designed to enhance your knowledge and sharpen your skills, are the perfect stepping stones to staying ahead of the curve in the dynamic world of surveying. We believe in the power of knowledge and its transformative potential. That's why our focus is on delivering comprehensive theoretical content that empowers you with a deep understanding of surveying principles. You won't find promises of "industry experts" or "practical skills" here. Instead, you'll embark on a journey of intellectual exploration guided by meticulously crafted course materials and thought-provoking insights. Immerse yourself in the world of surveying, and unlock limitless possibilities for career growth. Don't miss this extraordinary opportunity to fuel your passion for surveying and propel your career to new heights. Enrol in the Surveying (Building, Land and Development) QLS Endorsed bundle today, and become a force to be reckoned with in the surveying industry. Your success story starts here. Key Features of the Surveying (Building, Land and Development) QLS Endorsed Bundle: 3 QLS-Endorsed Courses: We proudly offer 3 QLS-endorsed courses within our Surveying (Building, Land and Development) QLS Endorsed bundle, providing you with industry-recognized qualifications. Plus, you'll receive a free hardcopy certificate for each of these courses. QLS Course 01: Building Quantity Surveyor QLS Course 02: Land Surveying QLS Course 03: Property Development Diploma 5 CPD QS Accredited Courses: Additionally, our bundle includes 5 relevant CPD QS accredited courses, ensuring that you stay up-to-date with the latest industry standards and practices. Course 01: Construction Estimation using RSMeans Course 02: LEED V4 - Building Design and Construction Course 03: WELL Building Standard Course 04: Construction Industry Scheme (CIS) Course 05: Bricklaying and Construction In Addition, you'll get Five Career Boosting Courses absolutely FREE with this Bundle. Course 01: Professional CV Writing Course 02: Job Search Skills Course 03: Self-Esteem & Confidence Building Course 04: Professional Diploma in Stress Management Course 05: Complete Communication Skills Master Class Convenient Online Learning: Our Surveying (Building, Land and Development) QLS Endorsed courses are accessible online, allowing you to learn at your own pace and from the comfort of your own home. Learning Outcomes: Develop a comprehensive understanding of building quantity surveying, land surveying, and property development principles. Acquire in-depth knowledge of construction estimation techniques using RSMeans. Gain proficiency in implementing LEED V4 - Building Design and Construction standards. Familiarize yourself with the WELL Building Standard and its application in sustainable construction practices. Understand the intricacies of the Construction Industry Scheme (CIS) and its impact on project management. Master the techniques of bricklaying and construction, enhancing your practical knowledge. Cultivate a strong foundation in surveying methodologies, enabling you to excel in your surveying career. Discover a world of limitless possibilities with the Surveying (Building, Land and Development) QLS Endorsed bundle. This comprehensive collection of eight courses provides aspiring surveying professionals with the essential knowledge and expertise needed to thrive in this dynamic field. With three QLS-endorsed courses, you'll receive hardcopy certificates, cementing your credibility and opening doors to exciting opportunities. Additionally, five CPD QS accredited courses will broaden your horizons, equipping you with the latest industry insights. Immerse yourself in theoretical exploration and embark on a transformative journey, laying the foundation for a successful career in surveying. CPD 250 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Surveying professionals seeking to enhance their knowledge and expertise. Individuals aspiring to pursue a career in building quantity surveying, land surveying, or property development. Construction industry personnel interested in expanding their skill set. Professionals looking to stay abreast of industry trends and best practices. Career path Building Quantity Surveyor: £30,000 to £60,000 per year. Land Surveyor: £25,000 to £50,000 per year. Property Developer: £35,000 to £80,000 per year. Construction Estimator: £25,000 to £50,000 per year. LEED Specialist: £35,000 to £65,000 per year. Construction Project Manager: £40,000 to £80,000 per year. Certificates Digital certificate Digital certificate - Included Hard copy certificate Hard copy certificate - Included
