- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
11447 Professional Development courses in Harpenden delivered Online
ISO 13485 Quality Management Foundation
By Training Centre
ISO 13485 Foundation training enables you to learn the basic elements to implement and manage a Medical Devices Quality Management System (MDQMS) as specified in ISO 13485. During this training course, you will be able to understand the different modules of a MDQMS, including MDQMS policy, procedures, performance measurements, management commitment, internal audit, management review and continual improvement. After completing this course, you can sit for the exam and gain "Certified ISO 13485 Foundation' Certification. This Certificate shows that you have understood the fundamental methodologies, requirements, framework and management approach. About This Course Learning Objectives Understand the elements and operations of a Medical Devices Quality Management System (MDQMS) Acknowledge the correlation between ISO 13485 and other standards and regulatory frameworks Understand the approaches, methods and techniques used for the implementation and management of a MDQMS Course Agenda Day 1: Introduction to Medical Devices Quality Management System (MDQMS) concepts as required by ISO 13485 Day 2: Medical Devices Quality Management System requirements and Certification Exam Assessment Delegates sit a combined exam, consisting of in-course quizzes and exercises, as well as a final 40 question, multiple choice exam on Day 2 of the course. The overall passing score is 70%, to be achieved within the 60 minute time allowance. Exam results are provided within 24 hours, with both a Certificate and a digital badge provided as proof of success. Prerequisites None What's Included? Certification fees are included on the exam price Training material containing over 200 pages of information and practical examples will be distributed An attestation of course completion worth 14 CPD (Continuing Professional Development) credits will be issued to the participants who have attended the training course. Who Should Attend? Individuals involved in Medical Devices Quality Management Individuals seeking to gain knowledge about the main processes of Medical Devices Quality Management Systems (MDQMS) Individuals interested to pursue a career in Medical Devices Quality Management Accreditation Provided by This course is Accredited by NACS and Administered by the IECB
Level 3 Health and Social Care with community worker Training
By Kingston Open College
Premium Bundle of all Time | Ofqual Regulation + ATHE Awards + CPD Accreditation | Assessment & Tutor Support Included

Overview This course will define the scope of work, project goal, project plan, project phase, sequencing and phase relationship Project Planning & Budgeting will provide participants with a demonstrated set of methods, processes, tools and techniques to cultivate a systematic and dynamic project plan to certify progressive monitoring control and reporting of the project cost.

Overview Tendering is a process on which a lot of money relies. When a tender is issued and published, winning that contract is completely depended on how deeply you understand the tender, the key areas of the tender along with how much in-depth knowledge you have about the potential client's need and how you can provide your service to those needs.

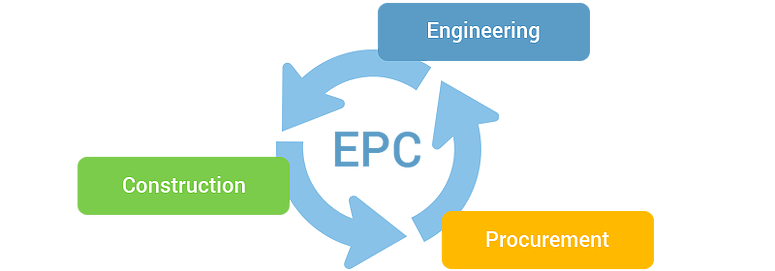
Overview EPC (Engineering, Procurement and Construction) is a very challenging area and is very competitive as well. Companies dealing with large and complex EPC projects are more often get involved in mitigation by complex contract laws and management that lead to huge financial losses. It is very important to Know-how EPC contract laws, and their commercial and financial aspects to gain skills and the ability to deal with complex contract laws and reduces the risk.
Overview The course focuses on topics such as the fundamental concepts of auditing and quality management, principles of internal and external audit, auditing processes and tools, principles and practice of root cause analysis, communication and people skills, and other related topics. Students who successfully complete this course will gain the essential knowledge and skills necessary to become successful auditors and work with confidence to improve the processes in their organizations.
Overview Strategic Financial Management and Effective Budget Execution also called Strategic Financial Management provide the important elements for attaining a comprehensive budget preparation and effective execution system. This course focuses on the risks and challenges likely to obstruct the operation of management and financial accounting processes and evaluates the techniques and tools needed to tackle them. It will highlight what constitutes strategic financial management and effective budget execution within the context of achieving their strategic and operational objectives.

Overview With the change in the density of enterprise risk, new risks have emerged, and managing it has become everyone's responsibility. The new Enterprise Risk Management course offers you the exclusive opportunity to learn the concepts and principles of the newly updated ERM framework and to integrate the framework into your organisation's strategy. The course is designed with all the modules to provide you with the knowledge necessary to understand and apply Enterprise Risk Management - Integrating with Strategy and Performance. The ERM framework assists management and boards of directors with their respective duties for managing risk.

Overview This course will help you manage project risk effectively by identifying, analyzing, and communicating inevitable changes to project scope and objectives. You will understand and practice the elements needed to measure and report on project scope, schedule, and cost performance. You will be equipped with the tools to manage change in the least disruptive way possible for your team and other project stakeholders.

