- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
11513 Professional Development courses in Durham delivered Online
VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

VAL05: Equipment Cleaning Validation
By Zenosis
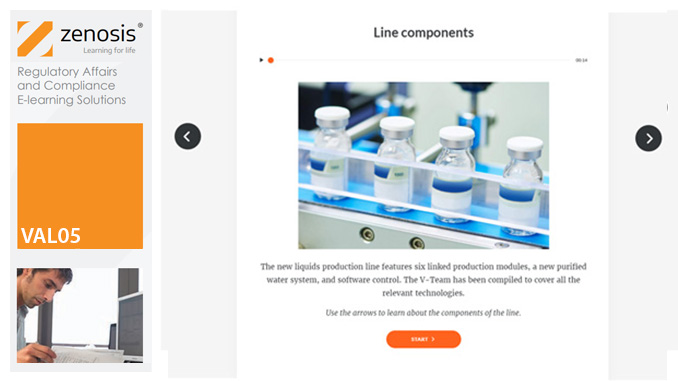
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.
VAL03: Commissioning and Installation Qualification
By Zenosis
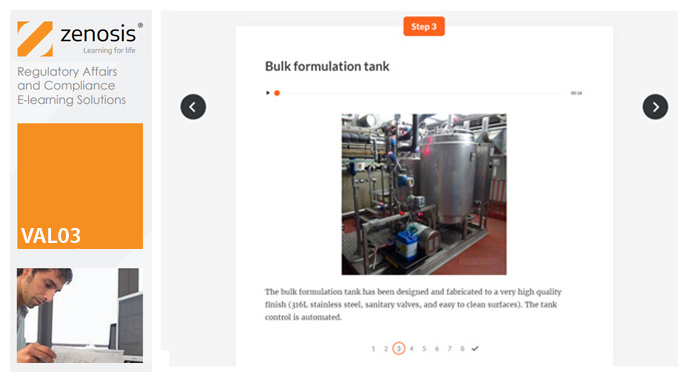
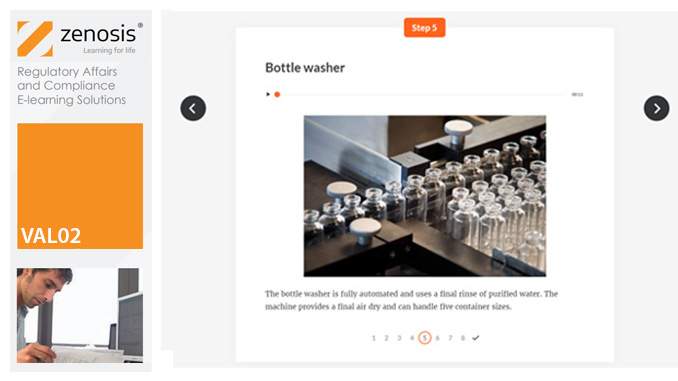
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
VAL04: Operational and Performance Qualification
By Zenosis
Having undergone Installation Qualification, before equipment can be used routinely in production, it needs to undergo Operational Qualification (OQ) and Performance Qualification (PQ). This module describes OQ and PQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

Project Management Diploma
By Study Plex
Recognised Accreditation This course is accredited by continuing professional development (CPD). CPD UK is globally recognised by employers, professional organisations, and academic institutions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. Course Curriculum Project Management Fundamentals: Know the Principles and Get it Right What is a Project 00:04:00 The Four Stage Project Lifecycle 00:08:00 Project Stages and Boundaries 00:08:00 One Reason why Projects go Wrong 00:05:00 Terminology used in the Project Stages 00:05:00 More on Project Gateways / Stage Gates 00:02:00 Project Definition Stage: The Vital Foundation to Your Success Define your Project: Goals and Objectives 00:10:00 Understanding Project Scope 00:06:00 Dealing with Scope Creep 00:06:00 Project Definition: Summary 00:03:00 Project Planning Stage: Failing to Plan = Planning to Fail The Book of the Plan 00:05:00 The Stakeholder Engagement Process 00:05:00 Stakeholder Analysis 00:07:00 Milestones are your Best Friends 00:08:00 The Work Breakdown Structure 00:08:00 The Gantt Chart 00:06:00 Tools for Creating a Gantt Chart 00:04:00 The Linear Responsibility Chart (LRC) aka The RACI Chart 00:09:00 The Risk Management Process 00:04:00 Risk Analysis 00:08:00 The Six Strategies for Managing Risks 00:09:00 The Risk Register (or Risk Log) 00:06:00 Project Delivery Stage: Don't you Love it When a Plan Comes Together! The Four Essentials of Leading your Team 00:07:00 Project Delivery - The Three Key Cycles 00:12:00 Project Closure Stage: Deep Sigh - You're Nearly Done Closing Words 00:01:00 Obtain Your Certificate Order Your Certificate of Achievement 00:00:00 Get Your Insurance Now Get Your Insurance Now 00:00:00 Feedback Feedback 00:00:00

