- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
10790 Professional Development courses in Pyle delivered On Demand
SUB01: Orphan Drug Designation in the USA and Europe
By Zenosis
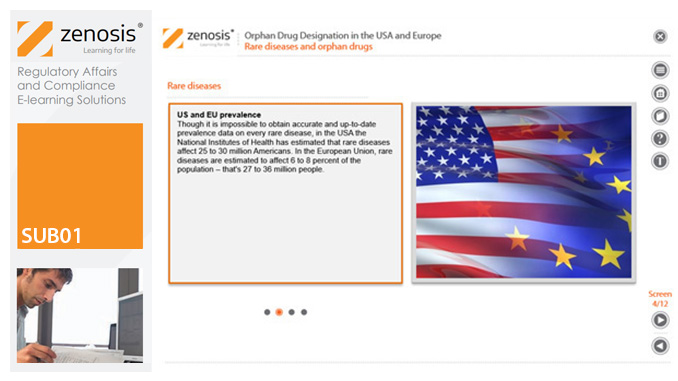
Medicines for the prevention, diagnosis, or treatment of rare diseases have become known as ‘orphan drugs’ because of their commercial unattractiveness. Development of such products is successfully encouraged through incentives offered by regulatory authorities. To qualify for important incentives, the sponsor of a drug must gain ‘orphan designation’ for its use in an indication. This module describes the requirements for orphan designation and how to apply for it in the USA and the European Economic Area.
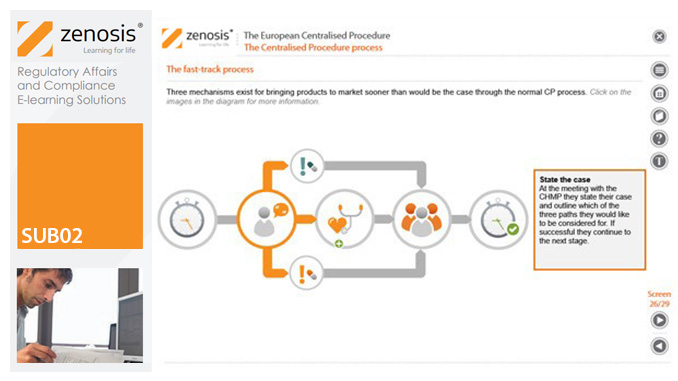
SUB02: The European Centralised Procedure (CP)
By Zenosis
The Centralised Procedure (CP) is one of three routes available to applicants to gain multinational marketing authorisation within the European Economic Area (EEA) on the basis of a single application. In the CP, one successful application leads to a marketing authorisation being issued by the European Commission that applies throughout the EEA. The CP is mandatory for certain types of products.
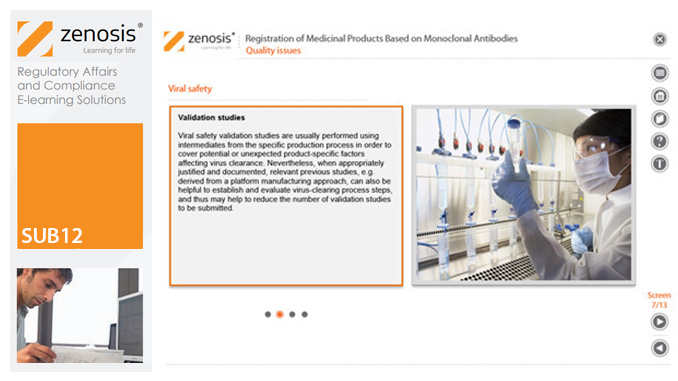
SUB12: Registration of Medicinal Products Based on Monoclonal Antibodies
By Zenosis
This module addresses characteristic issues influencing the registration of medicinal products based on monoclonal antibodies (mAbs), for use in humans. Regulatory requirements for the registration of biological medicinal products such as those based on mAbs differ in certain respects from those for small-molecule products. This is because of the distinct characteristics of biologics, such as complex structure and susceptibility to variation during manufacture.
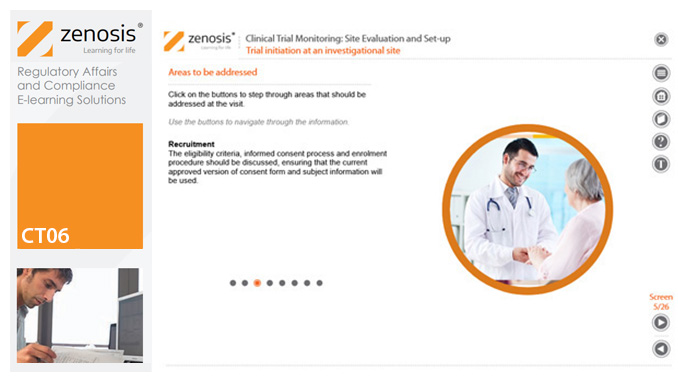
CT06: Clinical Trial Monitoring: Site Evaluation and Setup
By Zenosis
The sponsor of a clinical trial needs to reach agreement with clinical investigators to conduct the trial. The suitability of investigators and their institutional sites, typically hospitals, has to be evaluated, and the trial has to be set up at each site. This module describes the processes involved, focusing particularly on the role of a Clinical Research Associate (CRA) employed or contracted by the sponsor to monitor the trial.
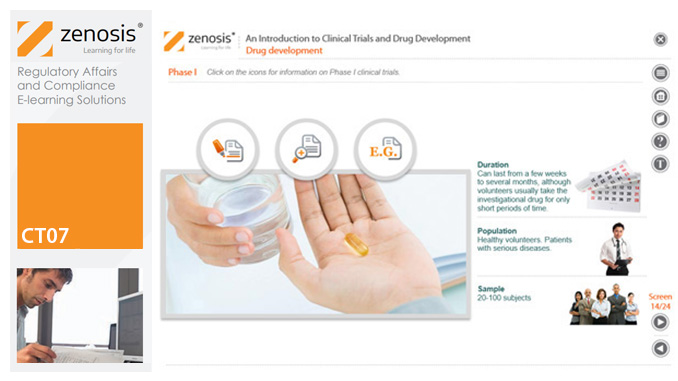
CT07: An Introduction to Clinical Trials and Drug Development
By Zenosis
This module provides an understanding of how clinical trials fit into the drug development process. It outlines the key historical events leading to the development of controlled clinical trials. It specifies the purpose of trials, outlines their features, and identifies codes and regulations that apply to them. Finally, it describes the environment of cost control in which the modern pharmaceutical industry operates.
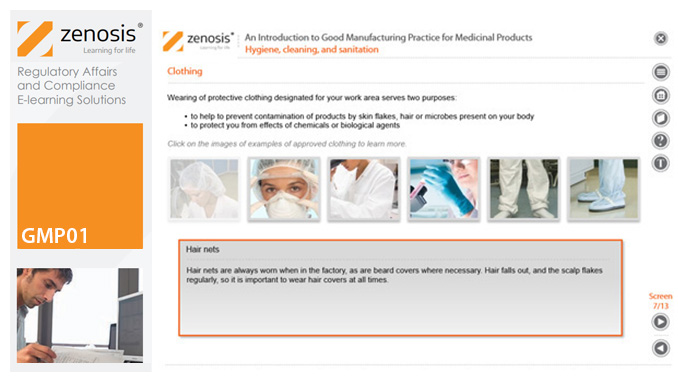
GMP01: An Introduction to Good Manufacturing Practice for Medicinal Products
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. The rules may be written into law or set out in guidance documents from regulatory authorities. Regulators will not allow medicinal products to be placed, or to remain, on the market in their country unless the products can be shown to be manufactured in compliance with GMP. To this end, they carry out inspections of manufacturing plants. Companies that persistently commit serious breaches of GMP requirements have suffered huge fines.
GMP04: Good Manufacturing Practice for the Warehouse
By Zenosis
The warehouse plays a crucial role in a medicinal products factory. This module explains the requirements of Good Manufacturing Practice (GMP) for the warehouse, and how to comply with them.

PKPD01: An Introduction to Pharmacokinetics and Pharmacodynamics in Drug Development and Registration
By Zenosis
Pharmacokinetic (PK) and pharmacodynamic (PD) studies provide a bridge between science and medicine in the development of a drug. In this module we describe the role of in-vivo PK and PD studies in a drug development programme, set out the uses to which the findings can be put, and discuss their implications for clinical development and application for marketing approval.

Level 3 Award for Special Educational Needs Coordinators in Early Years Settings
By Training Tale
This Level 3 Award for Special Educational Needs Coordinators in Early Years Settings course is designed for those interested in or who are currently in a SENCO role in the early years setting. This Level 3 Award for Special Educational Needs Coordinators in Early Years Settings qualification provides learners with a thorough understanding of the roles and responsibilities of the Special Educational Needs Coordinator in early years setting. Learn about the strategies and techniques for assisting children and their families and gain in-depth knowledge of SEN codes of practice. The purpose of this course is to help learners progress to further and higher education and develop new practical skills in health and social care. Detailed course curriculum Module 1: Roles and Responsibilities of the Special Educational Needs Coordinator in the Early Years Module 2: Strategies and Techniques for Supporting Children and Families Assessment Method After completing each module of the Level 3 Award for Special Educational Needs Coordinators in Early Years Settings, you will find automated MCQ quizzes. To unlock the next module, you need to complete the quiz task and get at least 60% marks. Once you complete all the modules in this manner, you will be qualified to request your certification. Certification After completing the MCQ/Assignment assessment for this Level 3 Award for Special Educational Needs Coordinators in Early Years Settings course, you will be entitled to a Certificate of Completion from Training Tale. It will act as proof of your extensive professional development. The certificate is in PDF format, which is completely free to download. A printed version is also available upon request. It will also be sent to you through a courier for £13.99. Who is this course for? This Level 3 Award for Special Educational Needs Coordinators in Early Years Settings course is ideal for those already working in a SENCo role as part of their Early Years Practitioner role or interested in doing so. This course is also suitable for childminders. Requirements There are no specific requirements for this Level 3 Award for Special Educational Needs Coordinators in Early Years Settings course because it does not require any advanced knowledge or skills. Certificates Certificate of completion Digital certificate - Included

The First Aid for Drivers (including baby and child First Aid) course is designed to give you the theoretical knowledge (and a lot more) needed to learn vital skills to be able to assist in road related medical injuries. This course is an excellent First Aid Training package for people to gain this invaluable knowledge as to how to help an adult, baby or child involved in a road traffic accident. The course covers topics such as CPR, the recovery position, choking, bleeds and so much more. The course consists of illustrated step by step directions, flow charts, diagrams, videos and test yourself sections fully compatible with all computers and mobile devices. You will be able to stop and start as often as you like and print your Certificate on completion. You will have continuous access to the course for 12 months. It is impossible to cover all eventualities within this course, or to equip you with the knowledge and skills to appropriately diagnose and treat in unpredictable real life situations. If you suspect serious illness or injury, you should always seek immediate professional medical advice. The Author has made every effort to ensure the accuracy of the information contained within the course, however this course is merely a guide and the Author does not accept any liability or responsibility for any inaccuracies or for any mistreatment or misdiagnosis of any person, however caused. The course material has been written by Emma Hammett, Qualified Nurse, First Aid Trainer and founder of First Aid for Life in conjunction with other medical and first aid professionals. If you have any queries concerning this course, please contact emma@firstaidforlife.org.uk Course contents: Action in an emergency Role of the First Aider The Primary Survey – How to help in an Emergency Unresponsive and Breathing Recovery Position Secondary Survey Heart Attack and Angina Unconscious and not breathing Resuscitation Breathing Problems Choking Asthma Panic Attacks and hyperventilation Anaphylactic Shock Wounds and bleeding Shock Fainting Burns Breaks, Sprains and Dislocations Head Injuries Spinal Injuries Log rolling someone into recovery position Major Crush injury – 15 minute rule Road Traffic Accidents Fitting / Seizures / Convulsions Extremes of body temperature Medical conditions Diabetes
