- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
31 Pivotal courses delivered Live Online
Unaccompanied Minors
By Immigration Advice Service
Discover the essentials of asylum for Unaccompanied Minors in our specialised course – navigating age determination, the asylum process, and decisions that impact their journey. View our Unaccompanied Minors training course dates below, available remotely via Microsoft Teams. Dive into the world of asylum with our extensive ‘Unaccompanied Minors’ course. Begin by understanding the fundamental definition of asylum and its pivotal role in providing protection to vulnerable individuals. Explore the unique challenges faced by unaccompanied minors, delving into the specific considerations that shape their asylum journey. Uncover the Home Office’s meticulous methods for determining the age of unaccompanied minors, a critical factor influencing the asylum process. Gain valuable insights into the step-by-step asylum claim procedure tailored specifically for this vulnerable group, highlighting the legal intricacies involved. Navigate the intricate web of asylum decisions, examining the factors that influence outcomes for unaccompanied minors. Explore the complexities that arise when age is disputed, delving into the procedures and considerations involved in resolving such disputes. This course is designed to empower professionals and advocates with comprehensive knowledge, enabling them to navigate the unique challenges of supporting and representing unaccompanied minors within the asylum framework. Be well-equipped to make informed decisions and provide effective assistance in ensuring the well-being and protection of these young individuals. Course joining links, materials and instructions are sent out 24hours before the course starts. Immigration Advice Service has been providing professional immigration services for over 10 years in the public, private and corporate sectors. Our fully qualified and OISC regulated trainer will guide you through the process and ensure you are competent in the area of Unaccompanied Minors. Who Should Attend: Designed for immigration Legal Practitioners, Lawyers, Solicitors, Immigration Advisors and Charities. Course Content: Brief definition of asylum Who are unaccompanied minors How Home Office determines age The process of asylum claims for unaccompanied minors Asylum decisions for unaccompanied minors What happens when age is disputed

Level 5 Learning & Development Consultant (Business Partner) - Coming Soon!
By Cavity Dental Training
Coming soon! Embark on a pioneering journey into the realm of strategic learning and development with our Level 5 Learning & Development Consultant (Business Partner) course. Tailored for individuals poised to step into the pivotal role of a Learning & Development Consultant, whether through recent recruitment or a well-deserved promotion, this program serves as a catalyst for acquiring advanced skills and unique insights crucial for excelling in the dynamic field of L&D.

Introduction to Diabetes
By BBO Training
Introduction to Diabetes (2-Day Course)Course Description:These two days of comprehensive training are designed for nurses, nurse associates, pharmacists, paramedics, and other Allied Healthcare Professionals (AHPs), and experienced healthcare assistants (HCAs) who are new to or fairly new to the field of diabetes care. If you've recently started seeing patients with diabetes, or are planning to; this course is tailored to provide you with the fundamental knowledge and skills required to confidently care for individuals with diabetes. The primary focus is on adults with Type 2 diabetes, although key recommendations and signposting for patients with Type 1 diabetes will also be covered.Diabetes presents a significant healthcare challenge, costing the NHS £10 billion each year and impacting patients and their families. Primary care professionals play a pivotal role in managing the ever-increasing numbers of people diagnosed with Type 2 diabetes. Good diabetes care is crucial and aligns with national and local policies supported by robust NICE guidance.These interactive days of learning will incorporate various methods, including case studies, to help you progress from basic knowledge to a more confident and positive approach in reviewing and managing patients with diabetes.Day One09.15 - Coffee and Registration09.30 - Introduction and Course Objectives09.45 - Setting the Scene: - Screening, Diagnosis, and Pre-Diabetes - Pathophysiology and Symptoms - Remission in Type 2 Diabetes10.45 - Coffee Break11.00 - Diet & Diabetes: - Healthy Eating - Carbohydrate Awareness - Range of Dietary Approaches - Weight Management Services01.00 - Lunch02.00 - Benefits of Activity02.15 - Foot Care and Diabetes02.45 - Organising Structured Diabetes Care in Practice: - QoF and the Annual Review - Care & Support Planning - 8 Care Processes and 3 Treatment Targets03.15 - Case Studies03.30 - Action Plan, Evaluation, and Resources03.45 - CloseDay Two09.15 - Coffee and Registration09.30 - Review Progress Since Day 109.45 - Non-Insulin Medications10.45 - Coffee Break11.00 - Medication Management01.00 - Lunch02.00 - Complications Associated with Diabetes02.45 - Hyperglycaemia, Hypoglycaemia, and Management of Illness03.15 - Blood Glucose and Ketone Monitoring03.30 - DVLA and Diabetes03.45 - Competencies, Training, Resources, and CloseKey Learning Outcomes for Both Days:Upon completing this course, participants will be able to:1. Explain the physiology of diabetes and differentiate between Type 1 and Type 2 diabetes.2. Discuss methods for diagnosing diabetes and provide information to individuals newly diagnosed with Type 2 diabetes.3. Describe approaches that support achieving remission in Type 2 diabetes.4. Explain basic advice related to a healthy diet, various dietary approaches, and carbohydrate awareness.5. Discuss the modes of action of commonly used non-insulin medications.6. Identify major complications that may arise in individuals with long-standing diabetes and measures to limit or prevent them.7. Describe key advice for patients regarding the recognition and appropriate treatment of hypoglycaemia.8. Discuss DVLA guidance concerning driving and diabetes.9. Explain the risks of acute hyperglycaemia and provide advice to patients on self-managing illness periods.10. Provide examples of referral pathways to other services such as weight management, secondary care, podiatry, structured education, activity, and psychological services.11. Describe the process of routine foot review and factors influencing diabetic foot risk status.12. Discuss local recommendations for the appropriate use of blood glucose and ketone monitoring.13. Explain the key components and processes of an annual diabetes review and a self-management plan.Join us for this comprehensive 2-day course via Zoom and enhance your ability to provide effective diabetes care within primary care settings.

Introduction to Good Manufacturing Practice
By Research Quality Association
Course Information This course offers foundational guidance and practical support tailored for individuals operating within Good Manufacturing Practice (GMP) frameworks. Explore the fundamental prerequisites of a pharmaceutical quality system (PQS) and delve into the application of quality risk management (QRM) principles, aligning with current regulations and guidance. Gain insights into pivotal aspects such as requirements, roles, and responsibilities, encompassing change control, document management, and key documentation essential for effective implementation of GMP with a focus on regulatory inspections and common findings. Is this course for you? Ideal for professionals engaged in GMP across various sectors, including: Research and Development (R&D) Contract Manufacturing Organisations Manufacturing Units Quality Control (QC) Laboratories Auditing Roles. What will you learn? Event objectives - by the end of the course, delegates shall: Have an awareness of the basic requirements of GMP Be aware of UK and EU GMP Rules and Guidance and relevant publications Understand the roles and responsibilities associated with GMP Be able to contribute to and maintain quality documentation Have a basic understanding of product lifecycle and manufacturing Understand the requirements of GMP in the QC laboratory context Have a basic understanding of risk management and mitigation principles Understand the need for quality systems and quality assurance activities Be aware of common regulatory findings. Learning outcomes: delegates will be able to: Implement their role within GMP with confidence and knowledge of the principle requirements Contribute effectively to the GMP quality system and their organisation’s compliance Comprehend where their organisation’s activities sit within the larger GMP arena Know where to seek further information within the published rules and guidance, UK Legislation, European Commission Directives, ICH Guidance and other relevant publications, as well as via the internet. Tutors Tutors will be comprised of (click the photos for biographies): Louise Handy Director, Handy Consulting Ltd Programme Please note timings may be subject to alteration. Day 1 09:30 Introductions and Scope of the Course Understand the group requirements and the tutor's background and experience. 09:45 Background and Regulatory Environment Setting the scene, understanding the context, key legislation. 10:30 Principles of GMP Key points and requirements. 11:15 Break 11:30 Personnel and Responsibilities Management and staff, duties and accountabilities. 12:00 Overview of GMP Manufacturing Basics of the product life cycle. 12:30 Lunch 13:15 Risk Management Workshop Practical exploration of risk and mitigation activities. 14:30 QC Laboratories Activities and practicalities. 15:15 Break 15:30 Compliance Quality Assurance and Self Inspection. 16:15 Question Time A chance for questions on the practicalities of GMP. 16:30 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Learn

The vital role of CFOs in business exit preparation
By FD Capital
he role of a CFO extends beyond day-to-day financial management and plays a pivotal role in preparing a business for an exit. The role of a CFO extends beyond day-to-day financial management and plays a pivotal role in preparing a business for an exit, whether it be through a merger, acquisition, or other strategic transaction. Here are some key points to consider: Financial Due Diligence: CFOs play a crucial role in conducting financial due diligence to assess the company’s financial health and identify any potential risks or issues. This involves reviewing financial statements, accounting practices, contracts, and other financial data to ensure accuracy and transparency. Valuation and Financial Modeling: CFOs work closely with the executive team, external advisors, and investment bankers to determine the company’s valuation. They develop financial models, assess growth projections, and analyze market comparables to arrive at a fair and realistic valuation range. Financial Documentation and Reporting: CFOs ensure that financial documentation and reporting are in order, accurate, and compliant with regulatory requirements. This includes preparing financial statements, management reports, and other financial disclosures necessary for the exit process. Negotiation and Deal Structuring: CFOs collaborate with legal and executive teams to negotiate the terms of the exit transaction. They provide financial insights and expertise to structure the deal in a way that maximizes value for the company and its stakeholders. Tax Planning and Optimisation: CFOs work closely with tax advisors to develop tax-efficient strategies for the exit transaction. They assess potential tax implications, explore tax-saving opportunities, and ensure compliance with applicable tax laws and regulations. Financial Communication and Investor Relations: CFOs play a critical role in communicating the financial aspects of the exit to internal and external stakeholders. They work with investor relations teams to ensure that key messages are effectively conveyed, providing transparency and clarity throughout the exit process. https://www.fdcapital.co.uk/podcast/the-vital-role-of-cfos-in-business-exit-preparation/ Tags Online Events Things To Do Online Online Seminars Online Business Seminars #business #cfo #preparation #exit #vital

VMware Tanzu RabbitMQ: Install, Configure, Manage
By Nexus Human
Duration 3 Days 18 CPD hours This course is intended for Developers Architects Administrators Overview By the end of the course, you should be able to meet the following objectives: Install and configure RabbitMQ Activate and use plugins such as the web management console Implement messaging patterns and applications using the Java client Set up a cluster of RabbitMQ nodes Configure high availability appropriately Tune and optimize RabbitMQ for better performance Secure RabbitMQ This intensive instructor-led course in RabbitMQ provides a deep dive into how to install, configure, and develop applications which leverage RabbitMQ messaging. The course begins with RabbitMQ installation and general configuration. It continues with developing messaging applications using the Java APIs, and delves into more advanced topics including clustering, high availability, performance, and security. Modules are accompanied by lab exercises that provide hands-on experience Introduction to Spring Essentials Kubernetes Overview BOSH Introduction Deploy, Patch & Upgrade Deploy a simple release Inside the VM Persistent Disks Patch the OS Upgrade Nginx Entry Point Set up a jumpbox Platform Infrastructure Pave the IaaS Deploy ops manager Deploy BOSH director Containerized Workloads Deploy Pivotal Container Service Provision a Kubernetes Cluster Harbor Container Registry Application Deployment Helm Advanced BOSH Deploy a distributed system Deploy Concourse CredHub Troubleshooting Concourse Deployment Concourse Day 2 Operations

Revit Courses Personalize Your Learning Schedule with On-Demand Options
By Real Animation Works
Personalized Revit Training: One-to-One Sessions with Pay-as-You-Go Flexibility

Own Your Success is delighted to bring you the 'Bitesize Masterclass' with Sophie Chapman. Not everyone has the flexibility to step away from the office for in-person events or dedicate extended periods from their packed schedules for full or half-day sessions. So we've put together our 'Bitesize Masterclasses' to help assistants develop and learn from professionals such as Sophie - shorter sessions, same big impact! Bitesize Masterclass with Sophie Chapman Sophie Chapman EA to Steven Bartlett Although Sophie initially aspired to be a talent agent, she swiftly realised that her career passions lay within the administrative/operational aspects of working life. Following her graduation in Entertainment Management from LIPA in 2008, she gained extensive work experience across renowned entities such as Sony BMG and Sky One, operating within the spheres of TV, music, radio, and the events industry. Her pivotal entry into a PA role came while supporting musician Eliza Doolittle. After five years, Sophie opted to move home to Liverpool and accept a contract supporting Sir Dave Brailsford of Team Sky. Once complete, Sophie received an offer for a position based in the US with an A-list musician. However, her journey led her to discover her "forever role", providing support to Steven Bartlett. Steven, the Founder and former Group CEO of Social Chain, is a multifaceted individual—a published author, podcaster, entrepreneur, and presently, the youngest-ever Dragon on BBC's Dragon's Den. Sophie has been instrumental as Steven's Executive Assistant, operating on a global scale, in both private and corporate realms for six years. Kate Wood Masterclass Facilitator Kate will aid the facilitation of this Bitesize Masterclass, ensuring that crucial topics are covered while prioritising an environment where every participant feels acknowledged, valued, and heard throughout the session. Kate is a business owner, qualified coach, consultant, facilitator, trainer, podcaster and public speaker with a background in learning & development, employee engagement strategy, continuous improvement, communications and marketing. After 25 years working across the board in blue-chip, non-profit and civil service organisations she now provides support to businesses looking to evolve and become great places for people to work in and with. Having worked with a wide variety of clients from school-age students to senior executives, she has been designing and running workshops, seminars and classes in person or via web events since she left long commutes and corporate roles behind in 2014 when she created her first two businesses. Her passion is improving working relationships between roles and functions and supporting people to discover their best selves at work. She does this in the automotive industry, financial services, medical teams, charities, global media, retail, education systems and the arts. When she’s not working, she and her classic car Monty Morgan can be found zipping around the English countryside. Session topics: How to establish your self-leadership position in your EA capacity Transition from a PA to EA Career development Boundary setting and wellbeing Q&A with the audience - Open-Book Q&A What is a Bitesize Masterclass? Short burst session. Keynote speakers discuss key themes/topics they are passionate about. Q&A with the audience attendees - ask the questions you don't usually get to ask! Run time of 90 minutes (max 2 hours) - we block out two hours in case we overspill on questions. 😊 Virtually delivered - attend from anywhere that's convenient for you! Packed with content, tips and tricks and from industry professionals who can share their experiences with you. Who will attend? PA's EA's Administrative roles --------------------------- At Own Your Success, we strongly believe in fostering an inclusive environment where diversity is celebrated and discrimination of any form is unwelcome. We welcome attendees from all backgrounds, regardless of race, ethnicity, gender identity, sexual orientation, religion, age, ability, or any other characteristic. Discrimination has no place in our workshops, and we are committed to creating a respectful and supportive space for all participants to learn and grow together. ---------------------------

10 practical ways to save time using ChatGPT and AI tools (In-House)
By The In House Training Company
ChatGPT, along with other AI tools, aims not to replace the human touch in management, but to enhance it. By addressing repetitive, daily tasks, these tools free up managers to concentrate on core responsibilities like strategic decision-making, team development, and innovation. As we move further into the digital age, integrating tools such as ChatGPT isn't a luxury; it's the future of proactive leadership. In this guide, we'll delve into 10 practical ways through which AI can elevate your efficiency and refine the quality of your work. Gain familiarity with prominent AI tools in the market Efficiently compose and respond to emails Generate concise summaries of complex reports and data. Obtain quick insights, data, and research across varied topics Streamline the writing of articles, training notes, and posts Craft interview tests, form relevant questions, and design checklists for the hiring process 1 Streamlining emails An inbox can be a goldmine of information but also a significant time drain for managers. Here's how to optimise it: Drafting responses: Give the AI a brief, and watch it craft a well-structured response. Sorting and prioritising: By employing user-defined rules and keywords, ChatGPT can flag important emails, ensuring no vital communication slips through the cracks. 2 Efficient report writing Reports, especially routine ones, can be time-intensive. Here's a smarter approach: Automate content: Supply key data points to the AI, and let it weave them into an insightful report. Proofreading: Lean on ChatGPT for grammar checks and consistency, ensuring each report remains crisp and error-free. 3 Rapid research From competitor insights to market trends, research is a pivotal part of management. Data synthesis: Feed raw data to the AI and receive succinct summaries in return. Question-answering: Pose specific questions about a dataset to ChatGPT and extract swift insights without diving deep into the entire content. 4 Reinventing recruitment Hiring can be a lengthy process. Here's how to make it more efficient: Resume screening: Equip the AI to spot keywords and qualifications, ensuring that only the most fitting candidates are shortlisted. Preliminary interviews: Leverage ChatGPT for the initial rounds of interviews by framing critical questions and evaluating the responses. 5 Enhancing training Especially for extensive teams, training can be a monumental task. Here's how ChatGPT can assist: Customised content: Inform the AI of your training goals, and it will draft tailored content suitable for various roles. PowerPoint design: Create visually appealing slide presentations on any topic in minimal time.

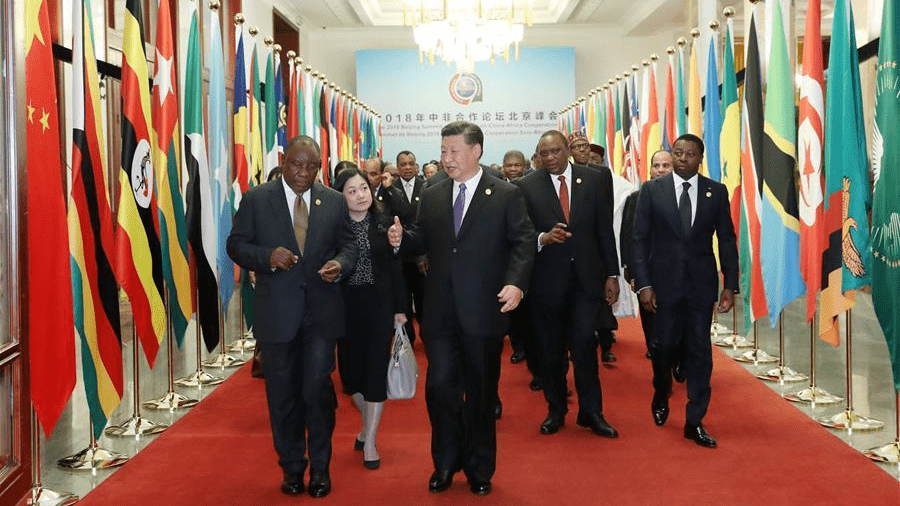
Global Power Shifts and China's Evolving Role in Africa | Live Online Learning
By Gada Academy
Explore China’s growing influence in Africa through this in-depth course. Weekly themes blend history, trends, and analysis to unpack the economic, political, and social layers of this evolving relationship. Gain a nuanced view of its impact on Africa’s global role