- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
286 Pharmacology courses delivered Online
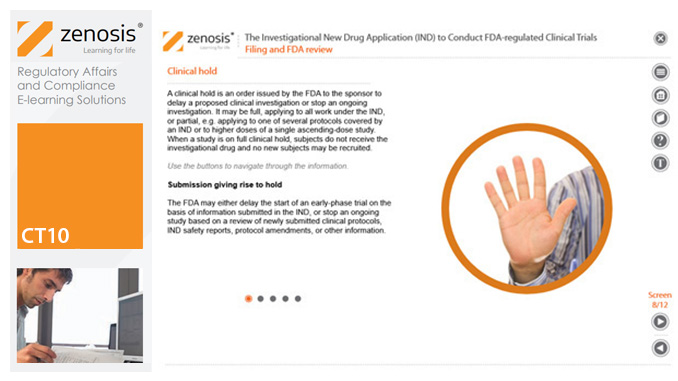
CT10: The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
By Zenosis
An Investigational New Drug Application (IND) is a submission to the US Food and Drug Administration (FDA) for permission to conduct a clinical trial of a medicinal product. This module describes regulatory requirements that sponsors or sponsor-investigators must meet for successful compilation, filing and maintenance of INDs. The IND and its role are defined, and the contexts in which it is required are specified.
CT03: ICH Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data. The International Council for Harmonisation’s guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice.

CT04: An Introduction to Clinical Trial Preparation and Design
By Zenosis
This module aims to provide you with effective strategies for the preparation and conduct of a clinical trial, while adhering to regulatory safety standards. Management of data for submission is also covered.

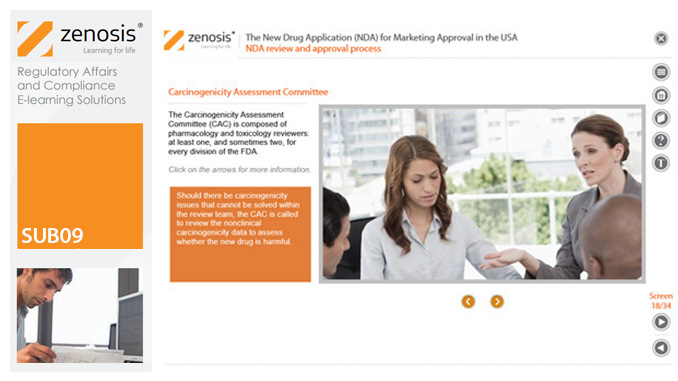
SUB09: The New Drug Application (NDA) for Marketing Approval in the USA
By Zenosis
The New Drug Application (NDA) is the regulatory vehicle through which sponsors formally propose that the Food and Drug Administration (FDA) approve a new pharmaceutical for marketing and sale in the USA.
CT09: Good Clinical Practice Inspections and Audits
By Zenosis
The module describes general principles of GCP inspection and audit, discusses preparation for an inspection, and sets out in detail what European and US FDA inspectors will examine. Finally it describes post-inspection actions by the regulator and the inspected party.

SUB05: Electronic Common Technical Document (eCTD)
By Zenosis
The eCTD is mandatory for all applications for marketing approval and all subsequent related submissions in the European Economic Area, the USA and Canada. Other countries intend to make its use mandatory. The eCTD specification has been developed to facilitate the global electronic submission, review and lifecycle management of medicinal product dossiers for regulatory applications. It broadens the scope of the CTD to include information on variations, renewals and amendments, so that it is no longer a static document but is updatable throughout the life of the product. This module outlines the eCTD specification, discusses the approach to regional differences in dossiers, and provides guidance on creation of an eCTD submission. The module provides a training and reference tool that will be of particular value to those new to the use of the format.

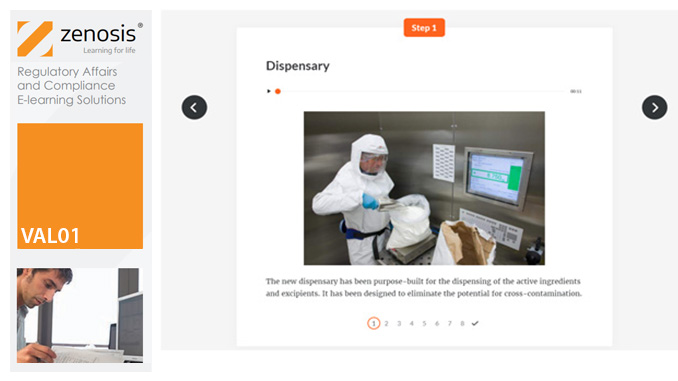
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL03: Commissioning and Installation Qualification
By Zenosis
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
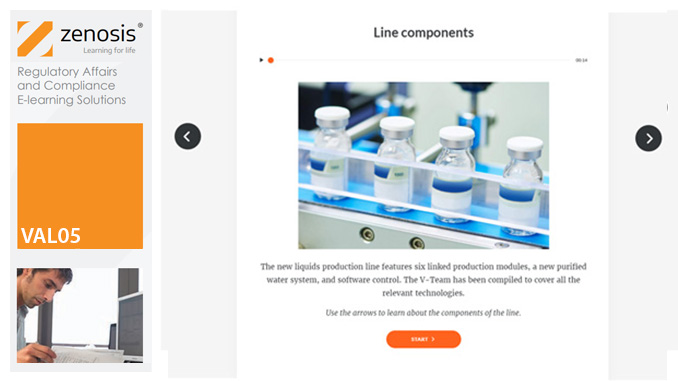
VAL05: Equipment Cleaning Validation
By Zenosis
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.