- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
4418 Pha courses
Overview This course is specially designed to enhance the skills required to analyse risk and crisis and the cause behind this. It will feed you with the necessary skills to minimise the chances of risk and in case it happens able to manage the situation in order to keep the business in a stable position. The course content will cover different crises caused due to internal or external issues and different ways to manage them. It will help the professionals to gain skills in planning beforehand various techniques to overcome risk and crisis and implementing the plan during that phase.

Network design training course description This course provides you with the knowledge needed to perform the design of a network infrastructure that supports desired network solutions to achieve effective performance, scalability, and availability. We recognise that the role of design does not normally require hands on skills but hands on sessions are used to reinforce the theory not to teach configuration or troubleshooting. What will you learn Create HA enterprise network designs. Develop optimum Layer 3 designs. Design effective modern WAN and data center networks. Develop effective migration approaches to IPv6. Create effective network security designs. Network design training course details Who will benefit: Anyone involved with network design. Prerequisites: TCP/IP Foundation for engineers Duration 5 days Network design training course contents Part I Reliable, resilient enterprise L2/3 network designOptimal Enterprise Campus Design:Enterprise campus design principles, hierarchy, modularity, flexibility, resiliency.EIGRP design:EIGRP Design, Should you use EIGRP?OSPF design: OSPF scalability designs, OSPF area design, OSPF Full-Mesh Design, OSPF Hub-and-Spoke Design, OSPF convergence design and optimization techniques. IS-IS Design:The protocol, IS-IS hierarchical architecture, IS-IS vs OSPF, IS-IS Deep Dive, IS-IS Design Considerations. BGP design:BGP overview, Designing Scalable iBGP Networks, BGP Route Reflector Design, Enhancing the Design of BGP Policies with BGP Communities, Case Study: Designing Enterprise wide BGP Policies Using BGP Communities, BGP Load-Sharing Design.Part II Enterprise IPv6 Design ConsiderationsIPv6 Design Considerations in the Enterprise: IPv6 Deployment and Design Considerations, Considerations for Migration to IPv6 Design, IPv6 Transition Mechanisms, Final Thoughts on IPv6 Transition Mechanisms. Challenges of the Transition to IPv6: IPv6 Services, Link Layer Security Considerations. Part III Modern Enterprise Wide-Area Networks DesignService Provider-Managed VPNs:Choosing Your WAN Connection, Layer 3 MPLS VPNs, Case Study: MPLS VPN Routing Propagation, Layer 2 MPLS VPN Services. Enterprise-Managed WANs: Enterprise-Managed VPNs, GRE, Multipoint GRE, Point-to-Point and Multipoint GRE, IPsec, IPsec and dynamic VTI, DMVPN, Case Study: EIGRP DMVPN, DMVPN and Redundancy, Case Study: MPLS/VPN over GRE/DMVPN, SSL VPN. Enterprise WAN Resiliency Design: WAN Remote-Site Overview, MPLS L3 WAN Design Models, Common L2 WAN Design Models, Common VPN WAN Design Models, 3G/4G VPN Design Models, Remote Site Using Local Internet, Remote-Site LAN, Case Study: Redundancy and Connectivity, NGWAN, SDWAN, and IWAN Solution Overview, IWAN Design Overview, Enterprise WAN and Access Management. Part IV Enterprise Data Center DesignsMultitier Data Center Designs: Case Study: Small Data Centers (Connecting Servers to an Enterprise LAN), Case Study: Two-Tier Data Center Network Architecture, Case Study: Three-Tier Data Center Network Architecture.Trends and Techniques to Design Modern Data Centers: The Need for a New Network Architecture, Limitations of Current Networking Technology, Modern Data Center Design Techniques and Architectures, Multitenant Data Center. SDN:SDN characteristics, How SDN addresses current Networking Limitations, SDN Architecture Components, SDN Network Virtualization overlays. Data Center Connections:Data Center Traffic Flows, The Need for DCI, IP Address Mobility, Case Study: Dark Fiber DCI, Pseudowire DCI. Part V Design QoS for Optimized User ExperienceQoS Overview:QoS Overview, IntServ versus DiffServ, Classification and Marking, Policers and Shapers, Policing Tools: Single-Rate Three-Color Marker, Policing Tools: TwoRate Three-Color Marker, Queuing Tools, Dropping Tools. QoS design principles and best practices: QoS overview, classification and marking design principles, policing and remarking design principles, queuing design principles, dropping design principles, Per-Hop behavior queue design principles, RFC 4594 QoS Recommendation, QoS Strategy Models. Campus QoS, WAN QoS, Data Center QoS.MPLS VPN QoS Design: The Need for QoS in MPLS VPN, Layer 2 Private WAN QoS Administration, Fully Meshed MPLS VPN QoS Administration, MPLS DiffServ Tunneling Modes, Sample MPLS VPN QoS Roles. IPsec VPN QoS Design: The Need for QoS in IPsec VPN, VPN Use Cases and Their QoS Models, IPsec Refresher, Encryption and Classification: Order of Operations, MTU Considerations, DMVPN QoS Considerations. Part VI IP Multicast DesignEnterprise IP Multicast Design: How Does IP Multicast Work? Multicast Protocols, Multicast Forwarding and RPF Check, Multicast Protocol Basics, PIM-SM Overview, Multicast Routing Table, Basic SSM Concepts, Bidirectional PIM. RP discovery, Anycast RP Features, MSDP. Part VII Designing Optimum Enterprise Network SecurityDesigning Security Services and Infrastructure Protection Network Security Zoning, Designing Infrastructure Protection.Designing firewall & IPS solutions: Firewall architectures, virtualized firewalls. Case Study: Application Tier separation, Case Study: Firewalls in a Data Center, Case Study: Firewall High Availability, IPS Architectures, Case Study: Secure Campus Edge Design (Internet and Extranet Connectivity). IP Multicast Security: Multicast Security Challenges, Multicast Network Security Considerations. Designing Network Access Control Solutions:IEEE 802.1X, EAP, 802.1X supplicants, 802.1X phased deployment, Case Study: Authorization Options. Part VIII Design scenariosDesign Case Studies: 1: Enterprise Connectivity, 2: Enterprise BGP with Internet Connectivity, 3: IPv6, 4: Data Center Connectivity, 5: Resilient Enterprise WAN, 6: Secure Enterprise Network, 7: QoS in the Enterprise Network.

Pharmacy Technician Complete Bundle - QLS Endorsed
By Imperial Academy
10 QLS Endorsed Courses for Pharmacy Technician | 10 Endorsed Certificates Included | Life Time Access

EC-Council Certified Cybersecurity Technician (C|CT)
By Nexus Human
Duration 5 Days 30 CPD hours This course is intended for The C|CT is ideal for anyone looking to start their career in cybersecurity or add a strong foundational understanding of the cybersecurity concepts and techniques required to be effective on the job. The course is especially well suited to: Early-career IT professionals, IT managers, career changers, and career advancers Students and recent graduates Overview After completing this course, you will understand: Key concepts in cybersecurity, including information security and network security Information security threats, vulnerabilities, and attacks The different types of malware Identification, authentication, and authorization Network security controls Network security assessment techniques and tools (threat hunting, threat intelligence, vulnerability assessment, ethical hacking, penetration testing, configuration and asset management) Application security design and testing techniques Fundamentals of virtualization, cloud computing, and cloud security Wireless network fundamentals, wireless encryption, and related security measures Fundamentals of mobile, IoT, and OT devices and related security measures Cryptography and public-key infrastructure Data security controls, data backup and retention methods, and data loss prevention techniques Network troubleshooting, traffic and log monitoring, and analysis of suspicious traffic The incident handling and response process Computer forensics and digital evidence fundamentals, including the phases of a forensic investigation Concepts in business continuity and disaster recovery Risk management concepts, phases, and frameworks EC-Council?s C|CT certification immerses students in well-constructed knowledge transfer. Training is accompanied by critical thinking challenges and immersive lab experiences that allow candidates to apply their knowledge and move into the skill development phase in the class itself. Upon completing the program, C|CT-certified professionals will have a strong foundation in cybersecurity principles and techniques as well as hands-on exposure to the tasks required in real-world jobs. Course Outline Information Security Threats and Vulnerabilities Information Security Attacks Network Security Fundamentals Identification, Authentication, and Authorization Network Security Controls: Administrative Controls Network Security Controls: Physical Controls Network Security Controls: Technical Controls Network Security Assessment Techniques and Tools Application Security Virtualization and Cloud Computing Wireless Network Security Mobile Device Security Internet of Things (IoT) and Operational Technology (OT) Security Cryptography Data Security Network Troubleshooting Network Traffic Monitoring Network Log Monitoring and Analysis Incident Response Computer Forensics Business Continuity and Disaster Recovery Risk Management

EC-Council Computer Hacking Forensic Investigator (CHFI) v10.0
By Nexus Human
Duration 5 Days 30 CPD hours This course is intended for The CHFI course will benefit: Police and other laws enforcement personnel Defense and Military personnel e-Business Security professionals Systems administrators Legal professionals Banking, Insurance and other professionals Government agencies Overview At the end of this course, you will possess the skills needed to: Understand the fundamentals of computer forensics Understand the computer forensic investigation process Describe in detail different types of hard disks and file systems Understand data acquisition and duplication Counteract anti-forensic techniques Leverage forensic skills in Windows, Linux, and Mac Investigate web attacks Understand dark web forensics Deploy forensic techniques for databases, cloud, and networks Investigate email crimes including malware Perform forensics in mobile and IoT environments Every crime leaves a digital footprint, and you need the skills to track those footprints. In this course, students will learn to unravel these pieces of evidence, decode them and report them. From decoding a hack to taking legal action against the perpetrators, they will become an active respondent in times of cyber-breaches. Computer Forensics in Today?s World 1.1. Understand the Fundamentals of Computer Forensics 1.2. Understand Cybercrimes and their Investigation Procedures 1.3. Understand Digital Evidence 1.4. Understand Forensic Readiness, Incident Response and the Role of SOC (Security Operations Center) in Computer Forensics 1.5. Identify the Roles and Responsibilities of a Forensic Investigator 1.6. Understand the Challenges Faced in Investigating Cybercrimes 1.7. Understand Legal Compliance in Computer Forensics Computer Forensics Investigation Process 2.1. Understand the Forensic Investigation Process and its Importance 2.2. Understand the Pre-investigation Phase 2.3. Understand First Response 2.4. Understand the Investigation Phase 2.5. Understand the Post-investigation Phase Understanding Hard Disks and File Systems 3.1. Describe Different Types of Disk Drives and their Characteristics 3.2. Explain the Logical Structure of a Disk 3.3. Understand Booting Process of Windows, Linux and Mac Operating Systems 3.4. Understand Various File Systems of Windows, Linux and Mac Operating Systems 3.5. Examine File System Using Autopsy and The Sleuth Kit Tools 3.6 Understand Storage Systems 3.7. Understand Encoding Standards and Hex Editors 3.8. Analyze Popular File Formats Using Hex Editor Data Acquisition and Duplication 4.1. Understand Data Acquisition Fundamentals 4.2. Understand Data Acquisition Methodology 4.3. Prepare an Image File for Examination Defeating Anti-forensics Techniques 5.1. Understand Anti-forensics Techniques 5.2. Discuss Data Deletion and Recycle Bin Forensics 5.3. Illustrate File Carving Techniques and Ways to Recover Evidence from Deleted Partitions 5.4. Explore Password Cracking/Bypassing Techniques 5.5. Detect Steganography, Hidden Data in File System Structures, Trail Obfuscation, and File Extension Mismatch 5.6. Understand Techniques of Artifact Wiping, Overwritten Data/Metadata Detection, and Encryption 5.7. Detect Program Packers and Footprint Minimizing Techniques 5.8. Understand Anti-forensics Countermeasures Windows Forensics 6.1. Collect Volatile and Non-volatile Information 6.2. Perform Windows Memory and Registry Analysis 6.3. Examine the Cache, Cookie and History Recorded in Web Browsers 6.4. Examine Windows Files and Metadata 6.5. Understand ShellBags, LNK Files, and Jump Lists 6.6. Understand Text-based Logs and Windows Event Logs Linux and Mac Forensics 7.1. Understand Volatile and Non-volatile Data in Linux 7.2. Analyze Filesystem Images Using The Sleuth Kit 7.3. Demonstrate Memory Forensics Using Volatility & PhotoRec 7.4. Understand Mac Forensics Network Forensics 8.1. Understand Network Forensics 8.2. Explain Logging Fundamentals and Network Forensic Readiness 8.3. Summarize Event Correlation Concepts 8.4. Identify Indicators of Compromise (IoCs) from Network Logs 8.5. Investigate Network Traffic 8.6. Perform Incident Detection and Examination with SIEM Tools 8.7. Monitor and Detect Wireless Network Attacks Investigating Web Attacks 9.1. Understand Web Application Forensics 9.2. Understand Internet Information Services (IIS) Logs 9.3. Understand Apache Web Server Logs 9.4. Understand the Functionality of Intrusion Detection System (IDS) 9.5. Understand the Functionality of Web Application Firewall (WAF) 9.6. Investigate Web Attacks on Windows-based Servers 9.7. Detect and Investigate Various Attacks on Web Applications Dark Web Forensics 10.1. Understand the Dark Web 10.2. Determine How to Identify the Traces of Tor Browser during Investigation 10.3. Perform Tor Browser Forensics Database Forensics 11.1. Understand Database Forensics and its Importance 11.2. Determine Data Storage and Database Evidence Repositories in MSSQL Server 11.3. Collect Evidence Files on MSSQL Server 11.4. Perform MSSQL Forensics 11.5. Understand Internal Architecture of MySQL and Structure of Data Directory 11.6. Understand Information Schema and List MySQL Utilities for Performing Forensic Analysis 11.7. Perform MySQL Forensics on WordPress Web Application Database Cloud Forensics 12.1. Understand the Basic Cloud Computing Concepts 12.2. Understand Cloud Forensics 12.3. Understand the Fundamentals of Amazon Web Services (AWS) 12.4. Determine How to Investigate Security Incidents in AWS 12.5. Understand the Fundamentals of Microsoft Azure 12.6. Determine How to Investigate Security Incidents in Azure 12.7. Understand Forensic Methodologies for Containers and Microservices Investigating Email Crimes 13.1. Understand Email Basics 13.2. Understand Email Crime Investigation and its Steps 13.3. U.S. Laws Against Email Crime Malware Forensics 14.1. Define Malware and Identify the Common Techniques Attackers Use to Spread Malware 14.2. Understand Malware Forensics Fundamentals and Recognize Types of Malware Analysis 14.3. Understand and Perform Static Analysis of Malware 14.4. Analyze Suspicious Word and PDF Documents 14.5. Understand Dynamic Malware Analysis Fundamentals and Approaches 14.6. Analyze Malware Behavior on System Properties in Real-time 14.7. Analyze Malware Behavior on Network in Real-time 14.8. Describe Fileless Malware Attacks and How they Happen 14.9. Perform Fileless Malware Analysis - Emotet Mobile Forensics 15.1. Understand the Importance of Mobile Device Forensics 15.2. Illustrate Architectural Layers and Boot Processes of Android and iOS Devices 15.3. Explain the Steps Involved in Mobile Forensics Process 15.4. Investigate Cellular Network Data 15.5. Understand SIM File System and its Data Acquisition Method 15.6. Illustrate Phone Locks and Discuss Rooting of Android and Jailbreaking of iOS Devices 15.7. Perform Logical Acquisition on Android and iOS Devices 15.8. Perform Physical Acquisition on Android and iOS Devices 15.9. Discuss Mobile Forensics Challenges and Prepare Investigation Report IoT Forensics 16.1. Understand IoT and IoT Security Problems 16.2. Recognize Different Types of IoT Threats 16.3. Understand IoT Forensics 16.4. Perform Forensics on IoT Devices

Certified Data Centre Expert (CDCE)
By Nexus Human
Duration 5 Days 30 CPD hours This course is intended for The primary audience for this course is any IT, facilities or data centre professional, who are involved in the design/build, renovation or relocation of a mission-critical data centre. Overview This 5-day course is designed to prepare participants to analyse a given business case and perform technical evaluation for a project plan and a set of designs for the implementation of a mission critical data centre. The course also engages participants in product evaluations and demonstrates how to select equipment and develop equipment test scripts (IET) and integrated performance and validation testing (IPVT). CDCE© builds upon knowledge gained in CDCP and CDCS courses. Participants who pass the exam will join the industry's elite data centre project design experts. CDCE© is the highest level training in the EPI Design and Build training track under the EPI Data Centre Training Framework. Participants must hold a valid CDCS certificate in order to be able to register for the CDCE class. CDCE© is the premier certification for data centre professionals in the data centre design/build and related fields. Data Centre Life Cycle Data centre lifecycle stages and phases Exercise: Stage/Phase/Milestone/Document mapping Design Preparation Creation of a SON ? Statement Of Need Technology review Conceptual sizing How to calculate for computer room space How to calculate facility space How to calculate incoming power Exercise: Conceptual sizing building and power Analysing capacity of existing facility Analysing investment options Site selection Permits and approvals Exercise: Site selection Conceptual design Budget and project timeline Business case preparation Project delivery structure Project management options Project manager and team Design Planning OSRA?Operational Systems Requirement Analysis TFRA?Technical Facilities Requirement Analysis Operations and maintenance review RFP?Request For Proposal process Vendor selection Design Development Project planning Design development PDR ? Preliminary Design Review Equipment selection FDR/V ? Final Design Review/Validation Exercise: Full design validation of power, cooling, floor plans, fire suppression Design freeze and LLTI Creation of construction documents BOM/BOQ ? Bill Of Material/Bill Of Quantity Exercise: Equipment selection Acquire Requirements of purchase orders Shipping terms FWT/FAT ? Factory Witness Test/Factory Acceptance Test Sequencing Incoming goods inspection and handling Asset management Construct Temporary essential services Erection of the building Permanent essential services Building inspection Snag list COF?Certificate Of Fitness Fit-Out Fit-Out Builders cleaning As-Built Drawings Test & Commissioning IET?Individual Equipment Test IPVT/IST?Integrated Performance Verification Test/Integrated Systems Test Common mistakes with IET/IPVT Deep cleaning Exercise: IET/IPVT scripting Hand-Over Facility hand-over requirements and documents PCC?Practical Completion Certificate DLP?Defect Liability Period Defect Management ICT Systems Installation ICT Systems Testing Hand-Over/DLP Expiry FCC?Final Completion Certificate Retirement Reasons and definitions of retirement Building the business case and project plan Sequencing Transfer of site Demolishing of site Legal matters FCC?Final Completion Certificate Exam: Certified Data Centre Expert (CDCE©) The CDCE© exam is in two parts: Part A is a 90-minute closed book exam, with 60 multiple-choice questions. For Part A, the candidate requires a minimum of 45 correct answers to pass the exam. Part B is a 90-minute closed book exam, with 25 open questions. For Part B the candidate needs to obtain a minimum of 75% to pass. Additional course details: Nexus Humans Certified Data Centre Expert (CDCE) training program is a workshop that presents an invigorating mix of sessions, lessons, and masterclasses meticulously crafted to propel your learning expedition forward. This immersive bootcamp-style experience boasts interactive lectures, hands-on labs, and collaborative hackathons, all strategically designed to fortify fundamental concepts. Guided by seasoned coaches, each session offers priceless insights and practical skills crucial for honing your expertise. Whether you're stepping into the realm of professional skills or a seasoned professional, this comprehensive course ensures you're equipped with the knowledge and prowess necessary for success. While we feel this is the best course for the Certified Data Centre Expert (CDCE) course and one of our Top 10 we encourage you to read the course outline to make sure it is the right content for you. Additionally, private sessions, closed classes or dedicated events are available both live online and at our training centres in Dublin and London, as well as at your offices anywhere in the UK, Ireland or across EMEA.

Advanced Process Safety Engineering
By EnergyEdge - Training for a Sustainable Energy Future
About this Training Course Managing process hazards in the hydrocarbon and chemical processing industries is a critical function that requires relevant knowledge and skills due to the risks involved. The Advanced Process Safety Engineering course will discuss the interrelation of the various techniques of process safety for analysing, with a particular emphasis on engineering design aspects, as well as how to manage process hazards in a safe and effective way and how they can potentially be avoided. In this 3 full-day advanced level course, the expert course leader will provide participants with insights and examples from his career and experience to show how their learning should be applied in real-life situations. Feedback and questioning is highly encouraged. Reference material and reports can be provided to give more information on any particular topic of interest. Individual and group exercises, tutored exercises and video case studies will be provided throughout the course to underpin the key learning points. Training Objectives Upon completion of this course, participants will acquire in-depth knowledge of: Risk management and 'As Low as Reasonably Practicable' (ALARP) principles. Different aspects of process design that influence process safety. Approach to 'inherently safer' design. Defence in depth using 'layers of protection'. Process for ensuring the technical integrity of safety-critical equipment. Hazards associated with process materials. Range of hazard identification and consequence modelling techniques. Causes and mitigation of human error. Reliability and availability of safety-critical protection equipment. Role of engineered safety-critical equipment and systems. Target Audience This course is suitable for industry professionals who need to acquire a comprehensive understanding of process safety. This includes those who are required to make managerial decisions where process safety is a key consideration, those who are moving into process safety positions or those who wish to broaden their process safety knowledge within their existing discipline. It is particularly suited for anyone involved in the design, operation, modification or maintenance of a major hazard installation, and will demonstrate a substantial understanding of process safety for those engaged in Continuous Professional Development or aiming for Chartered Engineer status. This course will benefit professionals such as: Operations and maintenance supervisors Process, mechanical and chemical engineers and technicians Design engineers, project engineers and HSE managers Control, automation and instrumentation engineers Course Level Advanced Trainer Your expert course leader has 50 years' experience in chemical and process safety engineering. His early career included 20 years in design and project engineering with various fine chemical and pharmaceutical companies where he designed chemical processes, specified plant equipment and selected materials for highly corrosive and toxic processes, often where textbook data was not available. This was followed by 10 years in offshore oil and gas design projects where he was responsible for setting up a Technical Safety group to change design safety practices in the aftermath of the 1988 Piper Alpha disaster. In recent years, he has been called upon to conduct various offshore and onshore incident investigations. His career has given him experience in project engineering, project management, process design and operations, safety engineering and risk management. He is a Fellow of the UK Institution of Chemical Engineers. He served on the Scottish Branch committee, and was elected chairman for a two-year term in 1991. He has also been chairman of the Safety and Reliability Society - North of Scotland Branch. He has delivered training courses in Process Hazard Analysis (HAZOP and HAZID), Process Safety Management, Hazard Awareness, Risk Assessment, Root Cause Analysis, Failure Modes & Effect Analysis and has lectured on Reliability Analysis to the M.Sc. course in Process Safety and Loss Prevention at Sheffield University. In addition to delivering training courses, he currently facilitates HAZOP / HAZID / LOPA studies and undertakes expert witness roles advising lawyers engaged in contractual disputes, usually involving the design or construction of chemical plants or Oil & Gas production facilities, or criminal prosecutions. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

CO2 Transportation From Capture to Storage and Usage
By EnergyEdge - Training for a Sustainable Energy Future
About this training course This 5-days comprehensive training course provides not only an introduction into the issues associated with the development of the pipeline transportation of CO2 from its Capture, it's transportation, storage and usage but also provides an in depth understanding of the issues to be considered in the development, design and operations of these pipelines. The theme throughout this training course is CO2 Flow Assurance and Innovative Technologies. Each training day will consist of lectures in the morning and a hands-on workshop in the afternoon. The workshop allows the participants to appreciate the design process associated with CO2 transport and its operations. Various software will be available during the workshop to predict thermo hydraulics and associated phase flows through CO2 pipelines and the attached infrastructure. Further software to assess surge and environmental safety will also be available. The CO2 pipeline design approach will consider an integrated solution through modelling the CO2 capture system as well as the host storage facility. The participants will have a total appreciation of the methodology required to develop a CO2 transportation system and have an understanding of all of the Flow Assurance, risks, and operating issues and technology requirements. Training Objectives After the completion of this training course, participants will be able to: Understand the process required and identify data to analyse Flow Assurance for CO2 pipeline transport Review and Identify the Flow Assurance issues required to be evaluated for CO2 pipeline transport design and operations methodology. These include; Hydrates, Slugging, Corrosion, Scaling, Fluid Phase Behaviour and transient conditions Establish the studies to be undertaken for each area of CO2 transport including 'Rules of Thumb' and software to be used Comprehend the need for innovative methods and the technologies to mitigate Flow Assurance issues and the need for economics considerations Appreciate the need for an integrated analysis of the CO2 transport system from the CO2 capture to the host storage facility Gain an appreciation of the emerging and enabling technologies for CO2 transport and storage application Dive deeper into the operational strategies requirements to mitigate Flow Assurance issues Target Audience This training course is suitable and will greatly benefit the following specific groups: Reservoir Engineers Flow Assurance Engineers Thermodynamics Engineers Process and Chemical Engineers Pipeline Engineers Facilities Engineers Control and Subsea Engineers working in the Oil and Gas industries Engineers in other disciplines may attend that require an appreciation of CO2 pipeline transport Control and Subsea Engineers working in the Oil and Gas industries Engineers in other disciplines may attend that require an appreciation of CO2 pipeline transport Course Level Intermediate Training Methods The training instructor relies on a highly interactive training method to enhance the learning process. This method ensures that all participants gain a complete understanding of all the topics covered. The training environment is highly stimulating, challenging, and effective because the participants will learn by case studies which will allow them to apply the material taught in their own organization. Course Duration: 5 days in total (35 hours). Training Schedule 0830 - Registration 0900 - Start of training 1030 - Morning Break 1045 - Training recommences 1230 - Lunch Break 1330 - Training recommences 1515 - Evening break 1530 - Training recommences 1700 - End of Training Course delivery: The maximum number of participants allowed for this training course is 20. A basic understanding of thermo-hydraulics would be advantageous. Morning Lectures and afternoon hands-on practical workshop. Special features: PC-based simulation software demonstration Workshop for hands-on training Course References & Additional resources: 'CO2 Transport from Capture to Storage and Usage', 1st Edition Handouts Pre & Post Assessment Trainer Your expert course leader is a renowned specialist in flow assurance management for the oil & gas field developments. His expertise enables him to support the operating and contracting companies as well as financial institutions regarding due diligence on offshore development investment decisions and associated operational system risks. Technical assessment of fields for acquisition and production enhancement opportunity. He possesses specialist expertise in evaluating difficult pipeline fluids transport such as Ethylene, Carbon Dioxide and Hydrogen through feasibility studies and technical reviews for clients. He has an exceptional academic background and natural leadership abilities are supported by practical experience of diverse projects worldwide, along with numerous publications at key conferences and publication of four books. Particular interests in developing novel and innovative technologies for subsea applications to solve difficult flow assurance problem areas and improve field development economics. He has worked on major industry projects including; Concorde aircraft fuelling system, the Channel Tunnel aerodynamics and the first deep water oil field development (Foinaven) in the West of Shetland. He is also currently developing a renewable energy solar farm and carbon neutral energy crop (Miscanthus) for domestic and commercial power generation application. He has developed in-house resources including specialist oil & gas field development evaluation software for subsea and onshore field infrastructure development options including; costing and financial analysis, reservoir viability, flow assurance assessment, subsea processing and boosting technologies, flow induced vibrations, surge analysis, heat transfer and chemical injection systems. Patents: · Subsea Seabed Power Generation for Depleting Gas fields Using Renewable Energy · Gas to Liquids Absorption Technology (GTLA) for subsea and onshore Flow Assurance applications · Subsea Gas Compressor System using pigs and liquid pumps · Pressure Boosting using water injection pumps · B&W Mentor subsea multi-phase meter · Surge suppression using a contained gas method for pipeline systems POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

EC-Council Certified Ethical Hacker (CEH) v12
By Nexus Human
Duration 5 Days 30 CPD hours This course is intended for The Certified Ethical Hacking v12 course will significantly benefit security officers, auditors, security professionals, site administrators, and anyone who is concerned about the integrity of the network infrastructure. Overview Information security controls, laws, and standards. Various types of footprinting, footprinting tools, and countermeasures. Network scanning techniques and scanning countermeasures Enumeration techniques and enumeration countermeasures Vulnerability analysis to identify security loopholes in the target organization?s network, communication infrastructure, and end systems. System hacking methodology, steganography, steganalysis attacks, and covering tracks to discover system and network vulnerabilities. Different types of malware (Trojan, Virus, worms, etc.), system auditing for malware attacks, malware analysis, and countermeasures. Packet sniffing techniques to discover network vulnerabilities and countermeasures to defend against sniffing. Social engineering techniques and how to identify theft attacks to audit human-level vulnerabilities and social engineering countermeasures. DoS/DDoS attack techniques and tools to audit a target and DoS/DDoS countermeasures. Session hijacking techniques to discover network-level session management, authentication/authorization, and cryptographic weaknesses and countermeasures. Webserver attacks and a comprehensive attack methodology to audit vulnerabilities in webserver infrastructure, and countermeasures. Web application attacks, comprehensive web application hacking methodology to audit vulnerabilities in web applications, and countermeasures. SQL injection attack techniques, injection detection tools to detect SQL injection attempts, and countermeasures. Wireless encryption, wireless hacking methodology, wireless hacking tools, and Wi-Fi security tools. Mobile platform attack vector, android vulnerability exploitations, and mobile security guidelines and tools. Firewall, IDS and honeypot evasion techniques, evasion tools and techniques to audit a network perimeter for weaknesses, and countermeasures. Cloud computing concepts (Container technology, serverless computing), the working of various threats and attacks, and security techniques and tools. Penetration testing, security audit, vulnerability assessment, and penetration testing roadmap. Threats to IoT and OT platforms and defending IoT and OT devices. Cryptography ciphers, Public Key Infrastructure (PKI), cryptography attacks, and cryptanalysis tools. CEH provides an in-depth understanding of ethical hacking phases, various attack vectors, and preventative countermeasures. It will teach you how hackers think and act maliciously so you will be better positioned to setup your security infrastructure and defend against future attacks. An understanding of system weaknesses and vulnerabilities helps organizations strengthen their system security controls to minimize the risk of an incident. CEH was built to incorporate a hands-on environment and systematic process across each ethical hacking domain and methodology, giving you the opportunity to work towards proving the required knowledge and skills needed to achieve the CEH credential. You will be exposed to an entirely different posture toward the responsibilities and measures required to be secure. Now in its 12th version, CEH continues to evolve with the latest operating systems, tools, tactics, exploits, and technologies. 1 - Introduction to Ethical Hacking Information Security Overview Cyber Kill Chain Concepts Hacking Concepts Ethical Hacking Concepts Information Security Controls Information Security Laws and Standards 2 - 2 - Foot-printing and Reconnaissance Footprinting Concepts Footprinting through Search Engines Footprinting through Web Services Footprinting through Social Networking Sites Website Footprinting Email Footprinting Who is Footprinting DNS Footprinting Network Footprinting Footprinting through Social Engineering Footprinting Tools Footprinting Countermeasures 3 - Scanning Networks Network Scanning Concepts Scanning Tools Host Discovery Port and Service Discovery OS Discovery (Banner Grabbing/OS Fingerprinting) Scanning Beyond IDS and Firewall Draw Network Diagrams 4 - Enumeration Enumeration Concepts NetBIOS Enumeration SNMP Enumeration LDAP Enumeration NTP and NFS Enumeration SMTP and DNS Enumeration Other Enumeration Techniques Enumeration Countermeasures 5 - Vulnerability Analysis Vulnerability Assessment Concepts Vulnerability Classification and Assessment Types Vulnerability Assessment Solutions and Tools Vulnerability Assessment Reports 6 - System Hacking System Hacking Concepts Gaining Access Escalating Privileges Maintaining Access Clearing Logs 7 - Malware Threats Malware Concepts APT Concepts Trojan Concepts Virus and Worm Concepts Fileless Malware Concepts Malware Analysis Countermeasures Anti-Malware Software 8 - Sniffing Sniffing Concepts Sniffing Technique: MAC Attacks Sniffing Technique: DHCP Attacks Sniffing Technique: ARP Poisoning Sniffing Technique: Spoofing Attacks Sniffing Technique: DNS Poisoning Sniffing Tools Countermeasures Sniffing Detection Techniques 9 - Social Engineering Social Engineering Concepts Social Engineering Techniques Insider Threats Impersonation on Social Networking Sites Identity Theft Countermeasures 10 - Denial-of-Service DoS/DDoS Concepts DoS/DDoS Attack Techniques BotnetsDDoS Case Study DoS/DDoS Attack Tools Countermeasures DoS/DDoS Protection Tools 11 - Session Hijacking Session Hijacking Concepts Application Level Session Hijacking Network Level Session Hijacking Session Hijacking Tools Countermeasures 12 - Evading IDS, Firewalls, and Honeypots IDS, IPS, Firewall, and Honeypot Concepts IDS, IPS, Firewall, and Honeypot Solutions Evading IDS Evading Firewalls IDS/Firewall Evading Tools Detecting Honeypots IDS/Firewall Evasion Countermeasures 13 - Hacking Web Servers Web Server Concepts Web Server Attacks Web Server Attack Methodology Web Server Attack Tools Countermeasures Patch Management Web Server Security Tools 14 - Hacking Web Applications Web Application Concepts Web Application Threats Web Application Hacking Methodology Web API, Webhooks, and Web Shell Web Application Security 15 - SQL Injection SQL Injection Concepts Types of SQL Injection SQL Injection Methodology SQL Injection Tools Evasion Techniques Countermeasures 16 - Hacking Wireless Networks Wireless Concepts Wireless Encryption Wireless Threats Wireless Hacking Methodology Wireless Hacking Tools Bluetooth Hacking Countermeasures Wireless Security Tools 17 - Hacking Mobile Platforms Mobile Platform Attack Vectors Hacking Android OS Hacking iOS Mobile Device Management Mobile Security Guidelines and Tools 18 - IoT and OT Hacking IoT Hacking IoT Concepts IoT Attacks IoT Hacking Methodology IoT Hacking Tools Countermeasures OT Hacking OT Concepts OT Attacks OT Hacking Methodology OT Hacking Tools Countermeasures 19 - Cloud Computing Cloud Computing Concepts Container Technology Serverless Computing Cloud Computing Threats Cloud Hacking Cloud Security 20 - Cryptography Cryptography Concepts Encryption Algorithms Cryptography Tools Public Key Infrastructure (PKI) Email Encryption Disk Encryption Cryptanalysis Countermeasures Additional course details: Nexus Humans EC-Council Certified Ethical Hacker (CEH) v.12 training program is a workshop that presents an invigorating mix of sessions, lessons, and masterclasses meticulously crafted to propel your learning expedition forward. This immersive bootcamp-style experience boasts interactive lectures, hands-on labs, and collaborative hackathons, all strategically designed to fortify fundamental concepts. Guided by seasoned coaches, each session offers priceless insights and practical skills crucial for honing your expertise. Whether you're stepping into the realm of professional skills or a seasoned professional, this comprehensive course ensures you're equipped with the knowledge and prowess necessary for success. While we feel this is the best course for the EC-Council Certified Ethical Hacker (CEH) v.12 course and one of our Top 10 we encourage you to read the course outline to make sure it is the right content for you. Additionally, private sessions, closed classes or dedicated events are available both live online and at our training centres in Dublin and London, as well as at your offices anywhere in the UK, Ireland or across EMEA.

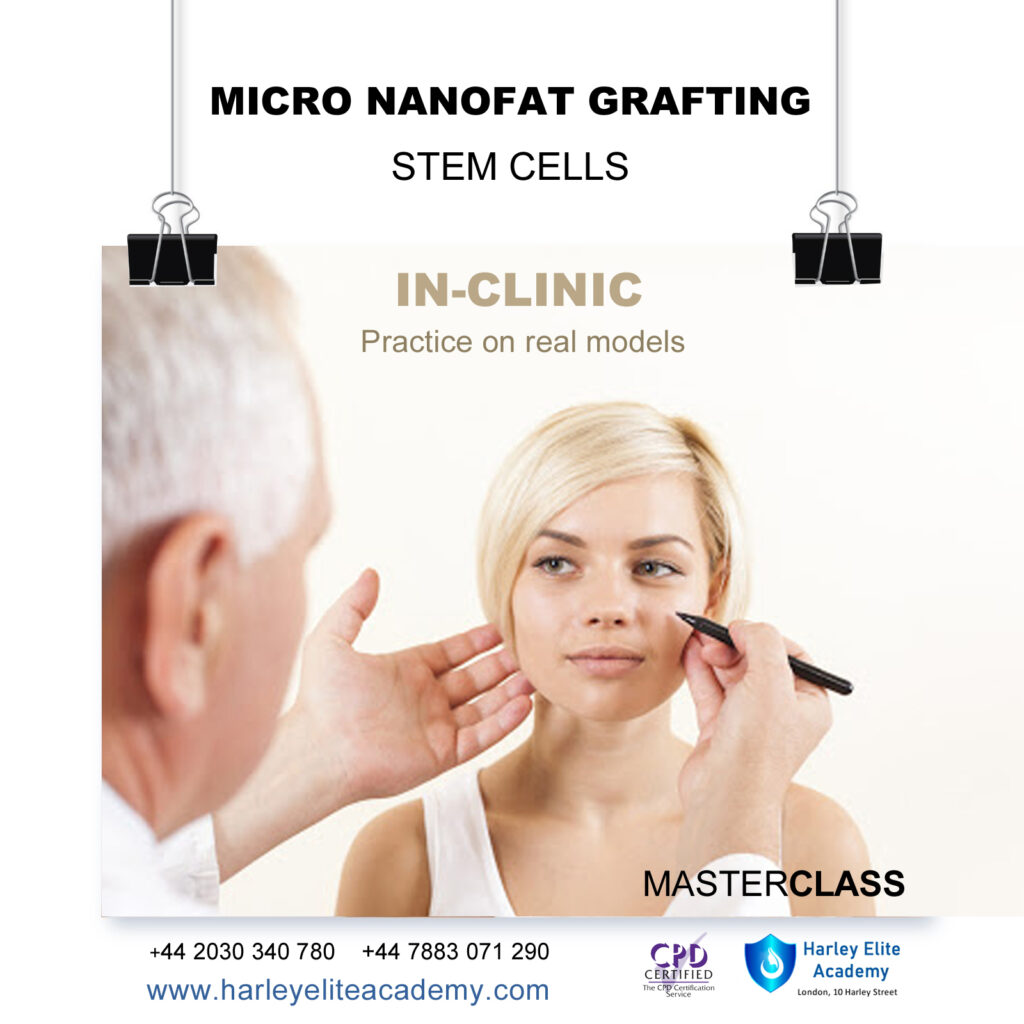
MICRO & NANOFAT GRAFTING TRAINING COURSE
By Harley Elite Academy (HeLa)
EXPERT – MASTERCLASS 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! DERMAGRAFT AND ENRICHED NANO-MICROFAT TRANSFER FOR FACE, NECK & HANDS – VOLUMIZATION & REJUVINATION WHAT IS MICROFAT AND NANOFAT GRAFTING FOR FACE AND NECK LIFT? Microfat and enriched nanofat graft transfer (also known as autologous fat transfer or lipoinjection), to the face and the neck is a minimally invasive, short-lasting procedure with rapid recovery, and can be done under local anaesthesia. Following completion of the procedure, the patient can return to the normal daily life activities. But firstly, we need to clearly explain what the terms microfat and nanofat grafts mean…. Microfat is defined a small-diameter fat particle and is used to improve the appearance of wrinkles, grooves and the lack of volume and sagginess of the skin of the face and neck, in response to the aging process. Similarly, nanofat, is referred to as the smallest diameter fat particle. The main advantage of the nanofat is that it contains cells that have the ability to differentiate in any type of body cell. Stem cells – as they are called- play a very important role in the process of rejuvenation, as they can differentiate into cells called fibroblasts which in turn produce collagen thus making the skin firmer and more youthful in appearance. The Course can be divided into two stages: 1. In the first stage, fat cells will be obtained from one or more parts of the body and are then purified and injected into the face and the neck. The doctor will identify an area in your body where the fat will be obtained from. This area is usually the flanks, but fat can also be obtained from other areas such as the abdomen, and the outer and inner thighs. You will be given enough local anaesthesia to numb the area where the fat will be harvested from. Then, a small-diameter cannula called microcannula connected to a special vacuum syringe will be gently introduced under the skin to obtain the fat. During this process, you should not feel any pain, the local anaesthetic will have made you numb. After the fat is harvested, it will contain a mixture of fat cells, blood, water, and local anaesthetic. Therefore, this fat will not be pure. This mixture of fat, blood, water and local anaesthetic will then become filtered into a special filtering and processing device whereby all the blood, water and local anaesthetic are being washed out, so that pure fat remains. This fat is then further processed by passing it through special filters whereby it becomes a smaller-sized fat particle known as microfat and nanofat graft. You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (theory), IN-CLINIC (Practice) COURSE LEVEL EXPERT | Masterclass Course
Search By Location
- Pha Courses in London
- Pha Courses in Birmingham
- Pha Courses in Glasgow
- Pha Courses in Liverpool
- Pha Courses in Bristol
- Pha Courses in Manchester
- Pha Courses in Sheffield
- Pha Courses in Leeds
- Pha Courses in Edinburgh
- Pha Courses in Leicester
- Pha Courses in Coventry
- Pha Courses in Bradford
- Pha Courses in Cardiff
- Pha Courses in Belfast
- Pha Courses in Nottingham