- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7576 Medicine & Nursing courses delivered Online
PKPD01: An Introduction to Pharmacokinetics and Pharmacodynamics in Drug Development and Registration
By Zenosis
Pharmacokinetic (PK) and pharmacodynamic (PD) studies provide a bridge between science and medicine in the development of a drug. In this module we describe the role of in-vivo PK and PD studies in a drug development programme, set out the uses to which the findings can be put, and discuss their implications for clinical development and application for marketing approval.

SUB01: Orphan Drug Designation in the USA and Europe
By Zenosis
Medicines for the prevention, diagnosis, or treatment of rare diseases have become known as ‘orphan drugs’ because of their commercial unattractiveness. Development of such products is successfully encouraged through incentives offered by regulatory authorities. To qualify for important incentives, the sponsor of a drug must gain ‘orphan designation’ for its use in an indication. This module describes the requirements for orphan designation and how to apply for it in the USA and the European Economic Area.
PKPD02: Conducting Pharmacokinetic and Pharmacodynamic Studies
By Zenosis
This module extends the learner’s understanding of pharmacokinetic and pharmacodynamic studies from the basics described in our companion module PKPD01, An Introduction to Pharmacokinetics and Pharmacodynamics in Drug Development and Registration. It provides detail on a variety of aspects of such studies: design, sampling, data analysis, research in special populations, and bioequivalence testing.

ICT01: Compliance with Regulation 21 CFR Part 11 on Electronic Records and Electronic Signatures
By Zenosis
21CFR11 applies to records that are required to be submitted to the FDA, or that are subject to FDA inspection, and that are in electronic form – that is, as computer files. It applies to all computer systems used to create, modify, maintain, archive, retrieve, or transmit such records – from a humble spreadsheet program to a complex information management system.

CT07: An Introduction to Clinical Trials and Drug Development
By Zenosis
This module provides an understanding of how clinical trials fit into the drug development process. It outlines the key historical events leading to the development of controlled clinical trials. It specifies the purpose of trials, outlines their features, and identifies codes and regulations that apply to them. Finally, it describes the environment of cost control in which the modern pharmaceutical industry operates.
SUB12: Registration of Medicinal Products Based on Monoclonal Antibodies
By Zenosis
This module addresses characteristic issues influencing the registration of medicinal products based on monoclonal antibodies (mAbs), for use in humans. Regulatory requirements for the registration of biological medicinal products such as those based on mAbs differ in certain respects from those for small-molecule products. This is because of the distinct characteristics of biologics, such as complex structure and susceptibility to variation during manufacture.
QUALIFI Level 7 Diploma in Psychology
By School of Business and Technology London
Getting Started The QUALIFI Level 7 Diploma in Psychology is designed to offer learners a comprehensive grasp of fundamental cognitive functions, including perception, executive functions, language, and attention, emphasising their significance within the realm of psychology. This course also focuses on equipping learners with practical skills essential for a successful career in psychology. Key Benefits Comprehensive Understanding: This qualification equips learners to explore the causes and development of mental health issues, including assessment and diagnosis. It takes a critical approach, considering biological risk factors and emphasising psychological and sociocultural factors. Statistical Proficiency: Students will develop a strong grasp of essential statistical techniques commonly used in psychological research. Practical application is facilitated through computer sessions where real-world research questions are presented, allowing students to use example data sets for analysis. Research Expertise: The qualification covers the entire research process, encompassing research techniques, ethical considerations, and scientific writing skills. The module "Issues in Scientific Research" introduces students to the intricate aspects of both theory and practice in research. Neuropsychological Insight: Learners will acquire knowledge of the neuropsychological dimensions of emotions and behaviour in healthy individuals and clinical populations. This provides valuable insights into the relationship between brain function and psychological processes. Career Pathways Upon successfully finishing the QUALIFI Level 7 Diploma in Psychology, learners can advance through further academic pursuits or by entering the workforce. Psychology Assistant with an estimated average salary of £26,473 per annum Mental Health Technician with an estimated average salary of £32,500 per annum Addiction Counselor with an estimated average salary of £37,593 per annum Behaviour Technician with an estimated average salary of £41,283 per annum About Awarding Body QUALIFI, recognised by Ofqual awarding organisation has assembled a reputation for maintaining significant skills in a wide range of job roles and industries which comprises Leadership, Hospitality & Catering, Health and Social Care, Enterprise and Management, Process Outsourcing and Public Services. They are liable for awarding organisations and thereby ensuring quality assurance in Wales and Northern Ireland. What is included? Outstanding tutor support that gives you supportive guidance all through the course accomplishment through the SBTL Support Desk Portal. Access our cutting-edge learning management platform to access vital learning resources and communicate with the support desk team. Quality learning materials such as structured lecture notes, study guides, and practical applications, which include real-world examples and case studies, will enable you to apply your knowledge. Learning materials are provided in one of the three formats: PDF, PowerPoint, or Interactive Text Content on the learning portal. The tutors will provide Formative assessment feedback to improve the learners' achievements. Assessment materials are accessible through our online learning platform. Supervision for all modules. Multiplatform accessibility through an online learning platform facilitates SBTL in providing learners with course materials directly through smartphones, laptops, tablets or desktops, allowing students to study at their convenience. Live Classes (for Blended Learning Students only) Assessment Time-constrained scenario-based assignments No examinations Entry Requirements The qualification aims to provide access without unnecessary barriers, and applicants should be at least 19 years old to apply. Non-native English speakers need an IELTS score of 6 or an equivalent language qualification. International applicants' qualifications will be checked for eligibility in UK higher education postgraduate programs. Additionally, applicants typically need to provide two references, with preference for academic concerns. Progression Achieving the QUALIFI Level 7 Diploma in Psychology provides learners with the opportunity to advance in the following ways: Pursuing the QUALIFI Level 8 Diploma. Enrolling in a university program to earn a Master's Degree. Entering into employment within a related profession. Why gain a QUALIFI Qualification? This suite of qualifications provides enormous opportunities to learners seeking career and professional development. The highlighting factor of this qualification is that: The learners attain career path support who wish to pursue their career in their denominated sectors; It helps provide a deep understanding of the health and social care sector and managing the organisations, which will, in turn, help enhance the learner's insight into their chosen sector. The qualification provides a real combination of disciplines and skills development opportunities. The Learners attain in-depth awareness concerning the organisation's functioning, aims and processes. They can also explore ways to respond positively to this challenging and complex health and social care environment. The learners will be introduced to managing the wide range of health and social care functions using theory, practice sessions and models that provide valuable knowledge. As a part of this suite of qualifications, the learners will be able to explore and attain hands-on training and experience in this field. Learners also acquire the ability to face and solve issues then and there by exposure to all the Units. The qualification will also help to Apply scientific and evaluative methods to develop those skills. Find out threats and opportunities. Develop knowledge in managerial, organisational and environmental issues. Develop and empower critical thinking and innovativeness to handle problems and difficulties. Practice judgement, own and take responsibility for decisions and actions. Develop the capacity to perceive and reflect on individual learning and improve their social and other transferable aptitudes and skills. Learners must request before enrolment to interchange unit(s) other than the preselected units shown in the SBTL website because we need to make sure the availability of learning materials for the requested unit(s). SBTL will reject an application if the learning materials for the requested interchange unit(s) are unavailable. Learners are not allowed to make any request to interchange unit(s) once enrolment is complete. To know about the Qualification Structure, please get in touch with us at: admission@sbusinesslondon.ac.uk Delivery Methods School of Business & Technology London provides various flexible delivery methods to its learners, including online learning and blended learning. Thus, learners can choose the mode of study as per their choice and convenience. The program is self-paced and accomplished through our cutting-edge Learning Management System. Learners can interact with tutors by messaging through the SBTL Support Desk Portal System to discuss the course materials, get guidance and assistance and request assessment feedbacks on assignments. We at SBTL offer outstanding support and infrastructure for both online and blended learning. We indeed pursue an innovative learning approach where traditional regular classroom-based learning is replaced by web-based learning and incredibly high support level. Learners enrolled at SBTL are allocated a dedicated tutor, whether online or blended learning, who provide learners with comprehensive guidance and support from start to finish. The significant difference between blended learning and online learning methods at SBTL is the Block Delivery of Online Live Sessions. Learners enrolled at SBTL on blended learning are offered a block delivery of online live sessions, which can be booked in advance on their convenience at additional cost. These live sessions are relevant to the learners' program of study and aim to enhance the student's comprehension of research, methodology and other essential study skills. We try to make these live sessions as communicating as possible by providing interactive activities and presentations. Resources and Support School of Business & Technology London is dedicated to offering excellent support on every step of your learning journey. School of Business & Technology London occupies a centralised tutor support desk portal. Our support team liaises with both tutors and learners to provide guidance, assessment feedback, and any other study support adequately and promptly. Once a learner raises a support request through the support desk portal (Be it for guidance, assessment feedback or any additional assistance), one of the support team members assign the relevant to request to an allocated tutor. As soon as the support receives a response from the allocated tutor, it will be made available to the learner in the portal. The support desk system is in place to assist the learners adequately and streamline all the support processes efficiently. Quality learning materials made by industry experts is a significant competitive edge of the School of Business & Technology London. Quality learning materials comprised of structured lecture notes, study guides, practical applications which includes real-world examples, and case studies that will enable you to apply your knowledge. Learning materials are provided in one of the three formats, such as PDF, PowerPoint, or Interactive Text Content on the learning portal. How does the Online Learning work at SBTL? We at SBTL follow a unique approach which differentiates us from other institutions. Indeed, we have taken distance education to a new phase where the support level is incredibly high.Now a days, convenience, flexibility and user-friendliness outweigh demands. Today, the transition from traditional classroom-based learning to online platforms is a significant result of these specifications. In this context, a crucial role played by online learning by leveraging the opportunities for convenience and easier access. It benefits the people who want to enhance their career, life and education in parallel streams. SBTL's simplified online learning facilitates an individual to progress towards the accomplishment of higher career growth without stress and dilemmas. How will you study online? With the School of Business & Technology London, you can study wherever you are. You finish your program with the utmost flexibility. You will be provided with comprehensive tutor support online through SBTL Support Desk portal. How will I get tutor support online? School of Business & Technology London occupies a centralised tutor support desk portal, through which our support team liaise with both tutors and learners to provide guidance, assessment feedback, and any other study support adequately and promptly. Once a learner raises a support request through the support desk portal (Be it for guidance, assessment feedback or any additional assistance), one of the support team members assign the relevant to request to an allocated tutor. As soon as the support receive a response from the allocated tutor, it will be made available to the learner in the portal. The support desk system is in place to assist the learners adequately and to streamline all the support process efficiently. Learners should expect to receive a response on queries like guidance and assistance within 1 - 2 working days. However, if the support request is for assessment feedback, learners will receive the reply with feedback as per the time frame outlined in the Assessment Feedback Policy.

24 Hour Flash Deal **25-in-1 Nursery Teacher Training: Fostering Early Childhood Development Mega Bundle** Nursery Teacher Training: Fostering Early Childhood Development Enrolment Gifts **FREE PDF Certificate**FREE PDF Transcript ** FREE Exam** FREE Student ID ** Lifetime Access **FREE Enrolment Letter ** Take the initial steps toward a successful long-term career by studying the Nursery Teacher Training: Fostering Early Childhood Development package online with Studyhub through our online learning platform. The Nursery Teacher Training: Fostering Early Childhood Development bundle can help you improve your CV, wow potential employers, and differentiate yourself from the mass. This Nursery Teacher Training: Fostering Early Childhood Development course provides complete 360-degree training on Nursery Teacher Training: Fostering Early Childhood Development. You'll get not one, not two, not three, but twenty-five Nursery Teacher Training: Fostering Early Childhood Development courses included in this course. Plus Studyhub's signature Forever Access is given as always, meaning these Nursery Teacher Training: Fostering Early Childhood Development courses are yours for as long as you want them once you enrol in this course This Nursery Teacher Training: Fostering Early Childhood Development Bundle consists the following career oriented courses: Course 01: Nursery Assistant / Teacher Certificate Course 02: Montessori Teaching Fundamentals: Principles and Practice Course 03: Early Years Primary Teaching Course 04: Lesson Planning for Teaching Course 05: Blended Learning Course for Teachers Course 06: Using Reggilio Emilia Approach in Early Childhood Course Course 07: Positive Handling in Schools Course 08: Nursery Manager Course 09: Classroom Behaviour Management Training Course 10: Primary School Teacher Training Course 11: Teaching Fundamentals: Make Concepts Easy to Understand Course 12: Planning, Delivery and Assessment in Teaching Course 13: Teaching & Homeschooling Course 14: Teaching Anybody Anything Course 15: Teach to Engage Students Course 16: SEND Teaching Course Course 17: Applied Behaviour Analysis (ABA) for Autism Spectrum Disorders Course 18: Self Regulation in Young Children Course 19: Improving School Attendance Course 20: Effective Strategies for Teaching Students with Learning Disabilities Course Course 21: Children's Literature in Education Course 22: Teaching Phonics Course 23: Speech and Language Therapy: Communication Disorders Course 24: Child Protection Officer: Safeguarding Children's Welfare Course Course 25: Effective Communication Techniques for Teachers and Trainers The Nursery Teacher Training: Fostering Early Childhood Development course has been prepared by focusing largely on Nursery Teacher Training: Fostering Early Childhood Development career readiness. It has been designed by our Nursery Teacher Training: Fostering Early Childhood Development specialists in a manner that you will be likely to find yourself head and shoulders above the others. For better learning, one to one assistance will also be provided if it's required by any learners. The Nursery Teacher Training: Fostering Early Childhood Development Bundle is one of the most prestigious training offered at StudyHub and is highly valued by employers for good reason. This Nursery Teacher Training: Fostering Early Childhood Development bundle course has been created with twenty-five premium courses to provide our learners with the best learning experience possible to increase their understanding of their chosen field. This Nursery Teacher Training: Fostering Early Childhood Development Course, like every one of Study Hub's courses, is meticulously developed and well researched. Every one of the topics is divided into Nursery Teacher Training: Fostering Early Childhood Development Elementary modules, allowing our students to grasp each lesson quickly. The Nursery Teacher Training: Fostering Early Childhood Development course is self-paced and can be taken from the comfort of your home, office, or on the go! With our Student ID card you will get discounts on things like music, food, travel and clothes etc. In this exclusive Nursery Teacher Training: Fostering Early Childhood Development bundle, you really hit the jackpot. Here's what you get: Step by step Nursery Teacher Training: Fostering Early Childhood Development lessons One to one assistance from Nursery Teacher Training: Fostering Early Childhood Developmentprofessionals if you need it Innovative exams to test your knowledge after the Nursery Teacher Training: Fostering Early Childhood Developmentcourse 24/7 customer support should you encounter any hiccups Top-class learning portal Unlimited lifetime access to all twenty-five Nursery Teacher Training: Fostering Early Childhood Development courses Digital Certificate, Transcript and student ID are all included in the price PDF certificate immediately after passing Original copies of your Nursery Teacher Training: Fostering Early Childhood Development certificate and transcript on the next working day Easily learn the Nursery Teacher Training: Fostering Early Childhood Development skills and knowledge you want from the comfort of your home CPD 250 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Nursery Teacher Training: Fostering Early Childhood Development training is suitable for - Students Recent graduates Job Seekers Individuals who are already employed in the relevant sectors and wish to enhance their knowledge and expertise in Nursery Teacher Training: Fostering Early Childhood Development Requirements To participate in this Nursery Teacher Training: Fostering Early Childhood Development course, all you need is - A smart device A secure internet connection And a keen interest in Nursery Teacher Training: Fostering Early Childhood Development Career path You will be able to kickstart your Nursery Teacher Training: Fostering Early Childhood Development career because this course includes various courses as a bonus. This bundle is an excellent opportunity for you to learn multiple skills from the convenience of your own home and explore Nursery Teacher Training: Fostering Early Childhood Development career opportunities. Certificates CPD Accredited Certificate Digital certificate - Included CPD Accredited e-Certificate - Free CPD Accredited Hardcopy Certificate - Free Enrolment Letter - Free Student ID Card - Free

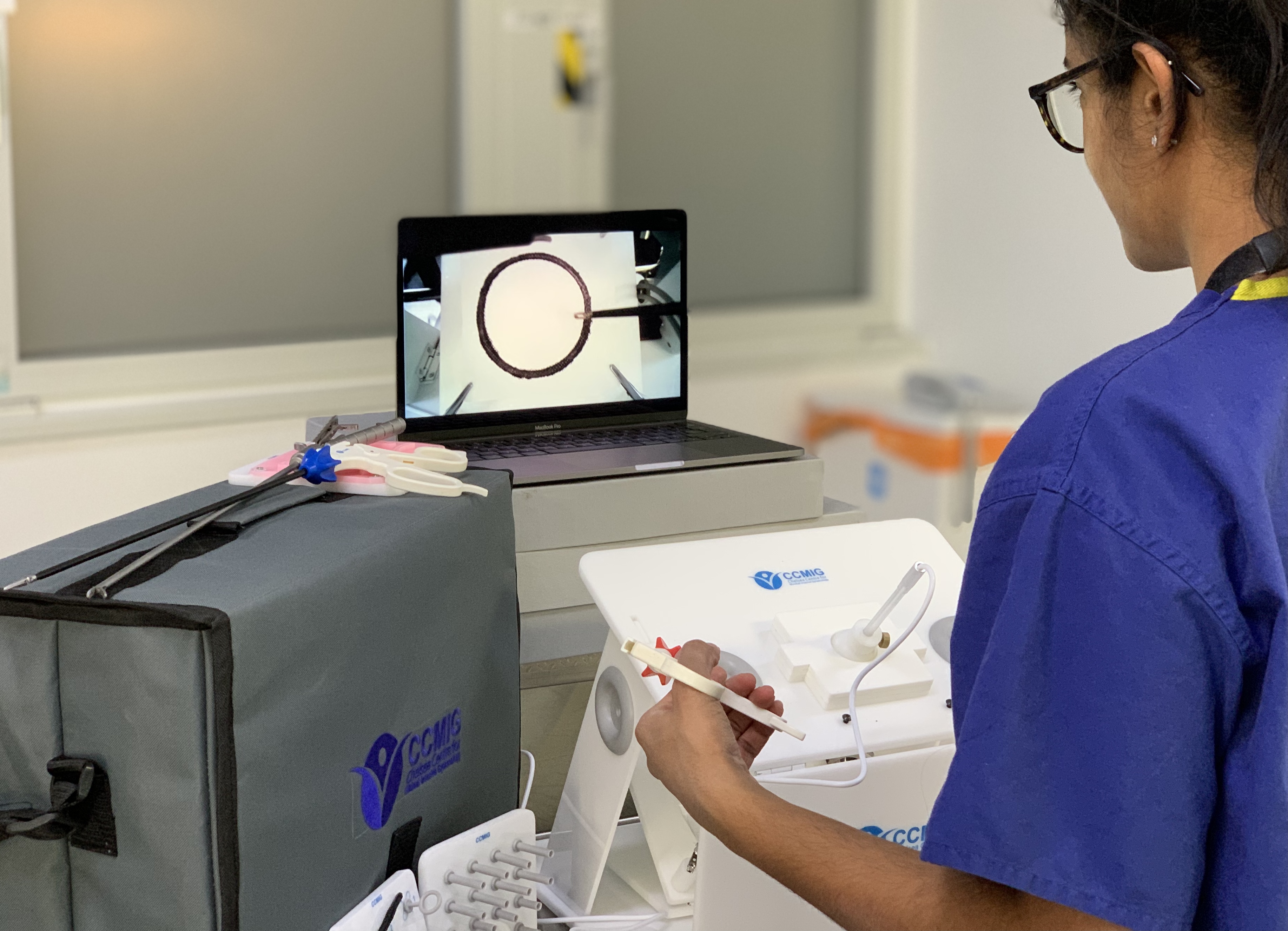
CCMIG Online Course
By CCMIG
Online modular laparoscopy course with theory and practical training. Assignments submitted for each module for progression on to the next.
Medical Receptionist and Medical Terminology Training - Double Endorsed Certificate
By Imperial Academy
2 QLS Endorsed Course | CPD Certified | Free PDF + Hardcopy Certificates | 80 CPD Points | Lifetime Access
