- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7445 Medicine & Nursing courses in Chigwell delivered Online
48-Hour Knowledge Knockdown! Prices Reduced Like Never Before! Sports therapists play a vital role in keeping athletes in peak condition. Did you know that in the UK alone, there are over 40,000 qualified sports therapists? This growing field offers excellent career opportunities for those passionate about sports and helping others. If you're passionate about sports and helping people heal, a career in sports therapy could be perfect for you. This Sports Therapy bundle offers a well-rounded education, from the fundamentals of anatomy and physiology to advanced techniques like pain management and sports massage. You'll delve into sports injuries, learning to identify, assess, and treat them effectively. The bundle also covers essential areas like sports nutrition, first aid, and sports medicine, giving you a holistic understanding of athlete care. With a single payment, you will gain access to Sports Therapy course, including 10 premium courses, a QLS Endorsed Hardcopy certificate (for the title course) and 11 PDF certificates for Absolutely free. This Sports Therapy Bundle Package includes: Main Course: Diploma in Sports Therapy at QLS Level 5 10 Additional CPDQS Accredited Premium Courses - Course 01: Sports Nutrition Course 02: Physiotherapy, Sports Massage and Reflexology Training Course 03: Anatomy and Physiology of Human Body Course 04: Sports Injuries Course 05: Pain Management Course 06: Shiatsu Massage for Beginners Course 07: Hydrotherapy Course 08: Sports Medicine Fundamentals Course 09: Sports First Aid Course 10: Thai Foot Reflexology Enrol today and take the first step towards a rewarding career in sports therapy. This course bundle empowers you to make a difference in the lives of athletes, helping them achieve their full potential. Learning Outcomes of Sports Therapy Apply anatomical and physiological knowledge to assess sports injuries. Implement physiotherapy and sports massage techniques for injury treatment. Develop personalized sports nutrition plans to optimize athletic performance. Design injury prevention strategies tailored to specific sports and athletes. Apply first aid principles to manage emergencies in a sporting environment. Demonstrate professionalism and ethical conduct in sports therapy practice. Why Choose Us? Get a Free QLS Endorsed Certificate upon completion of Sports Therapy Get a free student ID card with Sports Therapy Training program (£10 postal charge will be applicable for international delivery) The Sports Therapy is affordable and simple to understand This course is entirely online, interactive lesson with voiceover audio Get Lifetime access to the Sports Therapy course materials The Sports Therapy comes with 24/7 tutor support Start your learning journey straightaway! *** Course Curriculum *** Main Course: Diploma in Sports Therapy at QLS Level 5 Module 1: Introduction To Sports Therapy Module 2: Professionalism And Ethics Of Sports Therapy Module 3: Anatomy & Kinesiology Module 4: Assessment Of Sports Injuries Module 5: Sports Injuries Depend On Sports Profile Module 6: Common Sports Injuries Module 7: Major Sports Injure Module 8: First Aid Treatment Module 9: Sports Nutrition Module 10: Rehabilitation Exercise Module 11: Sports Massage Treatments Module 12: Hydrotherapy & Electrotherapy Course 01: Sports Nutrition Module 01: Introduction to Nutrition Module 02: The Role of Nutritionist Module 03: The Digestive System Module 04: Nutrients for Life - Macronutrients Module 05: Nutrients for Life - Micronutrients Module 06: Nutrition Requirements for Adults and Various Groups Module 07: Healthy Dieting Module 08: Weight Control Management Module 09: Weight Loss Management Module 10: The Day of Competition Module 11: Replacing Nutrients After Competition Course 02: Physiotherapy, Sports Massage and Reflexology Training Module 01: Introduction To Physiotherapy Module 02: The Standards For Physiotherapists Module 03: The Physiotherapy Interventions Module 04: Sports Physiotherapy Module 05: Neurological Physiotherapy Module 06: Musculoskeletal Physiotherapy Module 07: Respiratory Physiotherapy Module 08: Paediatric Physiotherapy Module 09: Evidence-Based Physiotherapy (EBP) Module 10: Building A Career As A Physiotherapist How will I get my Certificate? After successfully completing the course, you will be able to order your QLS Endorsed Certificates and CPD Accredited Certificates as proof of your achievement. PDF Certificate: Free (Previously it was £12.99*11 = £143) QLS Endorsed Hard Copy Certificate: Free (For The Title Course: Previously it was £119) CPD 255 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone interested in learning more about the topic is advised to take this bundle. This bundle is ideal for: Fitness Professionals Personal Trainers Sports Coaches Athletes Physiotherapists (PTs) Requirements You will not need any prior background or expertise to enrol in this bundle. Career path After completing this bundle, you are to start your career or begin the next phase of your career. Sports Massage Therapist Sports Therapist Physiotherapist Nutritionist Hydrotherapist Pain Management Specialist Certificates CPD Accredited Digital Certificate Digital certificate - Included Upon passing the Course, you need to order a Digital Certificate for each of the courses inside this bundle as proof of your new skills that are accredited by CPD QS for Free. Diploma in Sports Therapy at QLS Level 5 Hard copy certificate - Included Please note that International students have to pay an additional £10 as a shipment fee.

Neonatal Nursing, Midwifery, Paediatrics & Child Care - CPD Certified
4.7(47)By Academy for Health and Fitness
24-Hour Flash Sale! Prices Reduced Like Never Before!! 11 in 1 Neonatal Nursing, Midwifery, Paediatrics & Child Care - CPD Certified Bundle The healthcare sector of the UK is a beacon of excellence globally and here, the roles of neonatal nursing, midwifery, paediatrics, and child care are more crucial than ever. With over 600,000 births recorded annually and a significant portion of the population under 18, the demand for specialised healthcare professionals in these fields is not just substantial-it's growing. So, this "Neonatal Nursing, Midwifery, Paediatrics & Child Care - CPD Certified Bundle" is here to offer a comprehensive pathway for those ready to answer this call. This program offers a unique blend of courses, from specialised training in Neonatal Nursing, Midwifery, and Paediatrics to foundational courses in Child Care and nursing fundamentals. Whether you dream of delivering babies, caring for critically ill newborns, or nurturing young children in daycare settings, this bundle has you covered. The program also explores essential skills like Nurse Prescribing, Catheterisation, and Infection Control, ensuring you can provide safe and effective care. By getting into mental health nursing, you'll gain valuable insights into supporting children's emotional well-being. This Nursing Bundle Contains 11 of Our Premium Courses for One Discounted Price: Course 01: Neonatal Nursing Course 02: Midwifery Course 03: Paediatrics Course 04: Child Care Course 05: Nursing Assistant Training Course 06: Nurse Prescribing Diploma Course 07: Catheterisation Training for Nurse/Carer Course 08: Infection Control Course Course 09: Mental Health Nursing Level 3 Course 09: Medical Law Course 09: Risk Assessment & Care Management Stepping into the healthcare sector, especially in roles impacting neonatal, paediatric, and child care, is a journey of immense responsibility and profound reward. This bundle not only paves the way for a fulfilling career but also elevates your professional profile in a sector that shapes futures from the earliest stages of life. If you're driven by a passion to make a difference in the lives of children and families, now is the time to advance your skills, knowledge, and career with this comprehensive course bundle. Enrol now! Learning Outcomes of this Bundle: Acquire extensive knowledge of neonatal nursing care and practices. Understand holistic midwifery approaches for expectant mothers. Gain comprehensive knowledge of paediatric healthcare. Master foundational childcare techniques and strategies. Learn essential skills for nursing assistants. Understand nurse prescribing and infection control measures. Why Prefer this Course? Get a Free CPD Accredited Certificate upon completion of the course Get a Free Student ID Card with this training program (£10 postal charge will be applicable for international delivery) The course is Affordable and Simple to understand Get Lifetime Access to the course materials The training program comes with 24/7 Tutor Support Start your learning journey straight away! Course Curriculum Course 01: Neonatal Nursing Module 01: Introduction to Neonatal Nursing Module 02: Essential Care for the Newborn Module 03: Neonatal Assessment Module 04: Neonatal Resuscitation Module 05: Neonatal Infections Module 06: Neonatal Nutrition Module 07: Neonatal Anaesthesia Module 08: NICU Environment Course 02: Midwifery Module 01: Definition and Origin of Midwifery Module 02: Midwifery as a Career Option Module 03: Midwifery Management and Education Module 04: Midwivesââ¬â¢ Role in Health and Inequality Module 05: Social, Cultural and Spiritual Context of Childbearing Module 06: Antenatal Care and Screening Module 07: Midwifery Support in Labour: Intrapartum Care Module 08: Induction of labour Module 09: Postnatal Care as a Midwife Module 10: Nurture and Nature: The Healthy Newborn Module 11: Stillbirth, Neonatal Death and Bereavement Module 12: Infant feeding Module 13: Newborn Infection Module 14: Home Birth Module 15: Complementary and Alternative Medicines Applied to Maternity Care Module 16: Pharmacology and Medicines Management Module 17: Bleeding in Pregnancy Module 18: Medical Disorders of Pregnancy Module 19: Pre-term Labour Module 20: Twins and higher-order births Module 21: Care During the Third Stage of Labour Module 22: Terminology and Abbreviations for Midwifery Module 23: Perinatal mental health Module 24: Supporting a Healthy Pregnancy Module 25: Contraception and family planning Module 26: Effective Communication for Midwifery Module 27: Ethics in Midwifery Course 03: Paediatrics Module 01: Introduction to Paediatrician Module 02: Paediatric Nutrition and Nutritional Disorders Module 03: Neonatology Module 04: Gastroenterology Module 05: Haematology Module 06: Neurology Module 07: Immunology and Allergy Module 08: Oncology Module 09: Paediatrics, ethics, and the law =========>>>>> And 8 More Courses <<<<<========= How will I get my Certificate? After successfully completing the course, you will be able to order your Certificates as proof of your achievement. PDF Certificate: Free (Previously it was £12.99*11 = £143) CPD Hard Copy Certificate: Free (For The Title Course: Previously it was £29.99) CPD 110 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone interested in learning more about the topic is advised to take this bundle. This bundle is ideal for: Aspiring neonatal nurses Midwifery candidates Paediatric healthcare workers Child care providers Nursing assistants Nurse practitioners Please note: This course doesn't entitle you to practice as a professional in this specific field. Rather, this course will assist you in understanding the fundamentals so that you can improve your knowledge in the relevant field. Requirements You will not need any prior background or expertise to enrol in this bundle. Career path After completing this bundle, you are to start your career or begin the next phase of your career. Neonatal Nurse - £25,000 to £30,000 Midwife - £25,000 to £38,000 Paediatric Nurse - £25,000 to £30,000 Child Care Coordinator - £20,000 to £25,000 Nursing Assistant - £17,000 to £23,000 Mental Health Nurse - £25,000 to £30,000 Certificates CPD Accredited Digital Certificate Digital certificate - Included Upon passing the Course, you need to order a Digital Certificate for each of the courses inside this bundle as proof of your new skills that are accredited by CPD QS for Free. CPD Accredited Hard Copy Certificate Hard copy certificate - Included Please note that International students have to pay an additional £10 as a shipment fee.

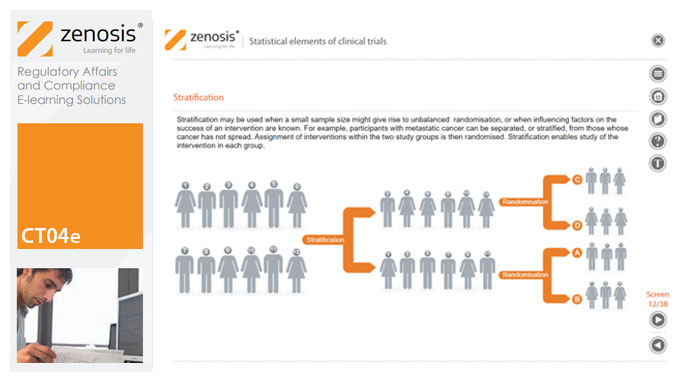
CT04e - Statistical elements of clinical trials
By Zenosis
Analytical statistical elements are essential concepts in the design of clinical trials. This analysis helps us to understand whether a conclusion from a study of a sample of the target population applies generally to that population as a whole. In particular, it helps us to answer the question: Did the treatment effect in the given study occur just by chance? The statistical elements of a well-controlled study minimise the chances of drawing the wrong conclusions, by providing clear thresholds for such errors. The basic statistical elements of a clinical trial include eligibility criteria, randomisation, sample size, power, and blinding, and these are discussed in this short course.
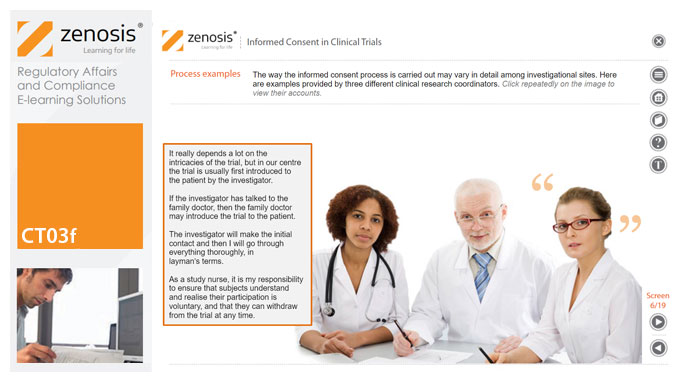
CT03f - Informed consent in clinical trials
By Zenosis
Informed consent in clinical research is an ethical and regulatory requirement. A research subject must enter a study voluntarily, be informed about risks and benefits, and understand the difference between investigation and treatment. Subjects must not be coerced into enrolment, nor must they be enticed by exaggerated claims of benefit. Before they can enrol, all potential subjects must agree, in writing, to participate. In addition to ethical and regulatory imperatives, the potential for litigation by subjects further highlights the importance of rigorous adherence to informed consent principles. In this short course we set out the principles and requirements and provide examples of practical issues confronting healthcare professionals and subjects.
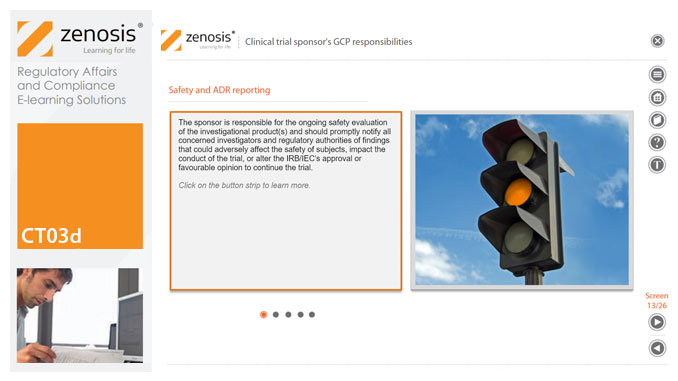
CT03d - Clinical trial sponsor’s GCP responsibilities
By Zenosis
The sponsor of a clinical trial takes responsibility for its initiation, management, and/or financing. A sponsor may transfer any or all of the sponsor’s trial-related duties and functions to a contract research organisation, but the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor. Duties and functions discussed in this short course include trial design, selection of investigators, QA and QC, data handling and record keeping, finance and compensation, regulatory submissions, management of investigational product(s), safety reporting, monitoring, audit, dealing with noncompliance, and clinical trial reports. ICH guideline E6 (revision 2) encourages sponsors to adopt a risk-based approach to managing the quality of trials. We discuss this approach in general, and aspects such as risk-based monitoring in particular.
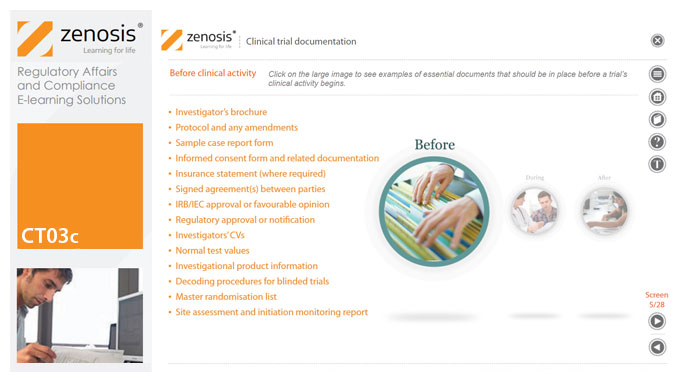
CT03c - Clinical trial documentation
By Zenosis
Regulatory authorities tend to abide by the maxim that ‘If it isn’t documented, it didn’t happen’. Rigorous documentation of all aspects of a clinical trial is necessary to provide evidence of GCP and compliance with regulatory requirements, as well as enabling effective management of the trial. In this short course we describe important examples of the documents designated by ICH GCP as essential to the conduct of a clinical trial.
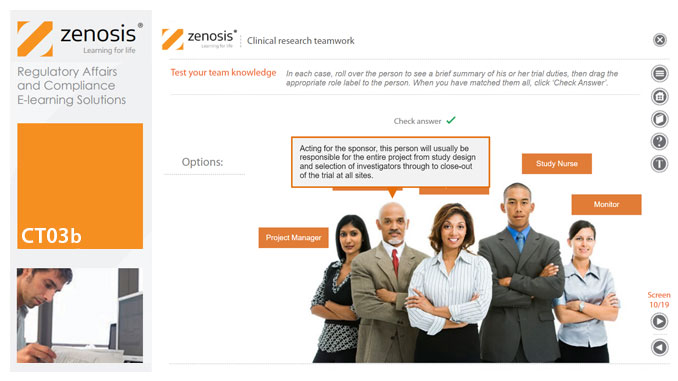
CT03b - Clinical research teamwork
By Zenosis
A clinical trial, particularly a late-phase commercial study, is a major project requiring collaboration between the sponsor and staff or contractor, on the one hand, and the clinical investigator(s) and other healthcare professionals on the other. Good communication among all parties is essential. In this short course we introduce the major roles in a typical clinical research project and outline their duties.
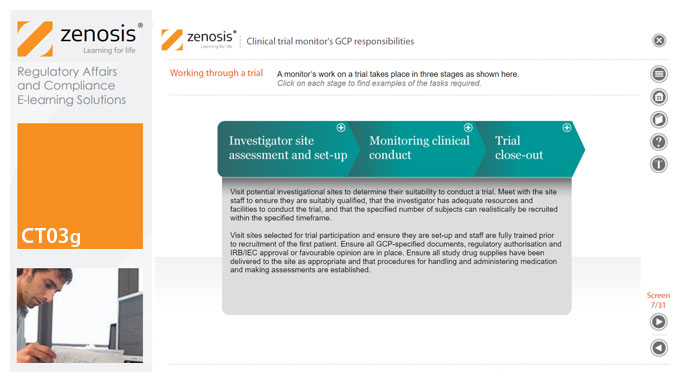
CT03g - Clinical trial monitor’s GCP responsibilities
By Zenosis
A clinical trial monitor acts on behalf of the sponsor to support investigational site personnel, verify the accuracy of data recorded, and ensure that the trial is conducted in compliance with the protocol, GCP and other study specific requirements. He or she acts as the ‘eyes and ears’ of the sponsor at the investigational site and provides the main channel of communication between sponsor and investigator. This short course explores the responsibilities of the monitor and provides insight into key challenges. We discuss assessment of investigators and investigational sites, education and trial initiation, monitoring of clinical conduct, including CRF review and source document verification, and trial close-out. We discuss noncompliance and how to deal with it.
CT04a - Clinical trials in drug development
By Zenosis
New drug development requires major investment in capital, human resources and technical expertise. Strict adherence to regulations on testing and manufacturing standards is also required before a new drug can be marketed. One of the greatest challenges in conducting clinical trials is that of efficiency. As trials become more comprehensive, involving large numbers of participants globally, their duration is prolonged and costs increase. The longer trials last, the shorter is the patent life remaining after market approval and the longer patients must wait for the new product. This short course covers the key components of clinical trials and how these requirements interact with the drug development cycle.

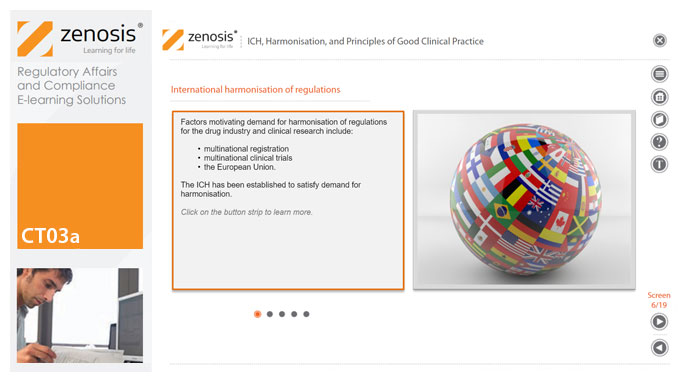
CT03a - ICH, harmonisation, and principles of Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data in applications for marketing approval. The International Council for Harmonisation's guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice. In this short course we describe the ICH’s role in the harmonisation of regulations, introduce its guideline E6, and set out the principles of GCP.