- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7114 Medicine & Nursing courses in Manchester delivered On Demand
Home School First Aid
By Creative Outdoors
First aid to cover the curriculum requirements and much, much more. 10 week bite sized learning course with tasks and knowledge self checks.

SAM04: Marketing of Prescription Drugs in the USA – Interactions with Healthcare Professionals
By Zenosis
The heaviest legal penalties imposed on drug companies concern interactions with healthcare professionals in the context of prescription drug marketing, notably for violations of the Anti-Kickback Statute and the False Claims Act. Monetary penalties have amounted to billions of dollars in some cases.

SAM02: Regulatory Requirements and Guidance on Advertising and Promotion of Prescription Drugs in the USA
By Zenosis
In this course we explain how to advertise and promote prescription drugs in various media, whether to healthcare professionals or consumers, in compliance with legal requirements and guidance from the FDA.

CT14: Clinical Trial Safety Reporting Requirements in the EU and USA
By Zenosis
This course sets out the legal and regulatory requirements for safety reporting in clinical trials of medicinal products under the jurisdictions of the European Union and the USA. It builds on the foundation laid by our companion course CT13, Safety Reporting in Clinical Trials, and provides greater detail of specific requirements in those jurisdictions.

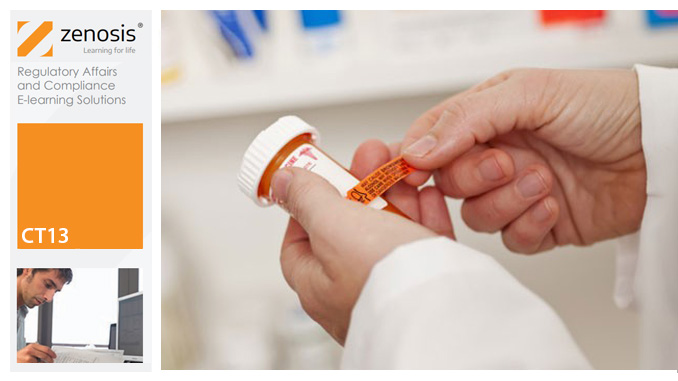
CT13: Safety Reporting in Clinical Trials
By Zenosis
This course explains the regulatory requirements for the reporting of adverse events and suspected adverse reactions in clinical trials. It describes how investigators should report to sponsors, and how sponsors should report to regulatory authorities and other stakeholders in the safety of investigational products. It explains how events are characterized as serious or non-serious, expected or unexpected, and it distinguishes the requirements for each category. It describes controlled vocabularies used for coding of events in reports.
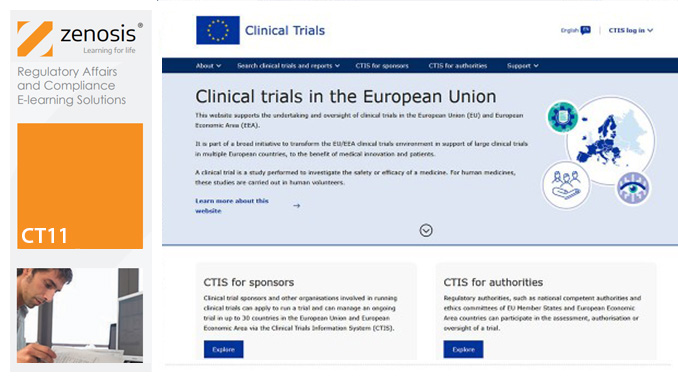
CT11: How to Gain Authorisation for Clinical Research Under the EU Clinical Trials Regulation
By Zenosis
This course sets out the procedures that sponsors need to follow to gain authorisation to conduct clinical trials under the Regulation, and it summarises and links to the extensive guidance available from the European Commission and the European Medicines Agency. Its companion course CT12 sets out the procedures that sponsors need to follow to conduct authorised clinical trials in compliance with the Regulation. The two courses therefore provide an ideal foundation for understanding and complying with the new law.
Level 3 Health and Social Care with Care Assistance Training Course
By Kingston Open College
Premium Bundle of all Time | Ofqual Regulation + ATHE Awards + QLS Endorsement | Assessment & Tutor Support Included

ATHE Awards Level 3 Health and Social Care - Regulated Qualification
By Kingston Open College
Premium Bundle of all Time | Ofqual Regulation + ATHE Awards + CPD Accreditation | Assessment & Tutor Support Included

Health & Social Care, Medical Law & Public Health
By Imperial Academy
3 QLS Endorsed Diploma | QLS Hard Copy Certificate Included | 10 CPD Courses | Lifetime Access | 24/7 Tutor Support

Physiology & Pain Management, First Aid at Work with (AED)
By Imperial Academy
Level 7, 5 & 3 QLS Endorsed Diploma | QLS Hard Copy Certificate Included | Plus 10 CPD Courses | Lifetime Access