- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7115 Medicine & Nursing courses in Harpenden delivered On Demand
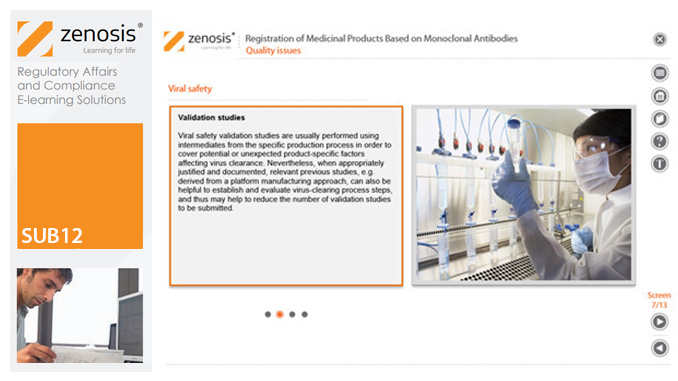
SUB12: Registration of Medicinal Products Based on Monoclonal Antibodies
By Zenosis
This module addresses characteristic issues influencing the registration of medicinal products based on monoclonal antibodies (mAbs), for use in humans. Regulatory requirements for the registration of biological medicinal products such as those based on mAbs differ in certain respects from those for small-molecule products. This is because of the distinct characteristics of biologics, such as complex structure and susceptibility to variation during manufacture.
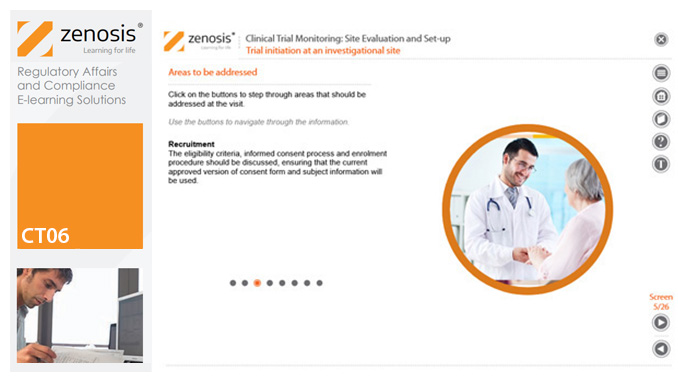
CT06: Clinical Trial Monitoring: Site Evaluation and Setup
By Zenosis
The sponsor of a clinical trial needs to reach agreement with clinical investigators to conduct the trial. The suitability of investigators and their institutional sites, typically hospitals, has to be evaluated, and the trial has to be set up at each site. This module describes the processes involved, focusing particularly on the role of a Clinical Research Associate (CRA) employed or contracted by the sponsor to monitor the trial.
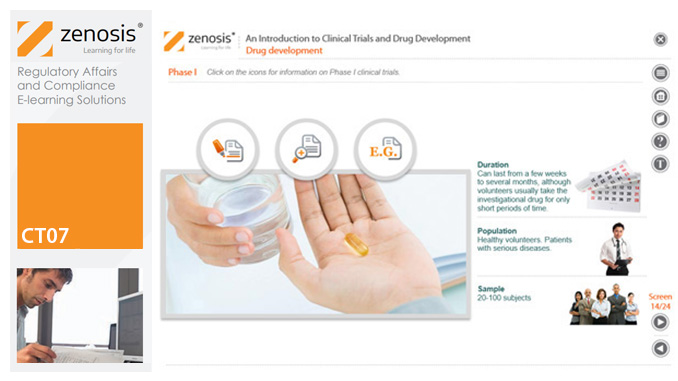
CT07: An Introduction to Clinical Trials and Drug Development
By Zenosis
This module provides an understanding of how clinical trials fit into the drug development process. It outlines the key historical events leading to the development of controlled clinical trials. It specifies the purpose of trials, outlines their features, and identifies codes and regulations that apply to them. Finally, it describes the environment of cost control in which the modern pharmaceutical industry operates.
GMP01: An Introduction to Good Manufacturing Practice for Medicinal Products
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. The rules may be written into law or set out in guidance documents from regulatory authorities. Regulators will not allow medicinal products to be placed, or to remain, on the market in their country unless the products can be shown to be manufactured in compliance with GMP. To this end, they carry out inspections of manufacturing plants. Companies that persistently commit serious breaches of GMP requirements have suffered huge fines.
GMP04: Good Manufacturing Practice for the Warehouse
By Zenosis
The warehouse plays a crucial role in a medicinal products factory. This module explains the requirements of Good Manufacturing Practice (GMP) for the warehouse, and how to comply with them.

PKPD01: An Introduction to Pharmacokinetics and Pharmacodynamics in Drug Development and Registration
By Zenosis
Pharmacokinetic (PK) and pharmacodynamic (PD) studies provide a bridge between science and medicine in the development of a drug. In this module we describe the role of in-vivo PK and PD studies in a drug development programme, set out the uses to which the findings can be put, and discuss their implications for clinical development and application for marketing approval.

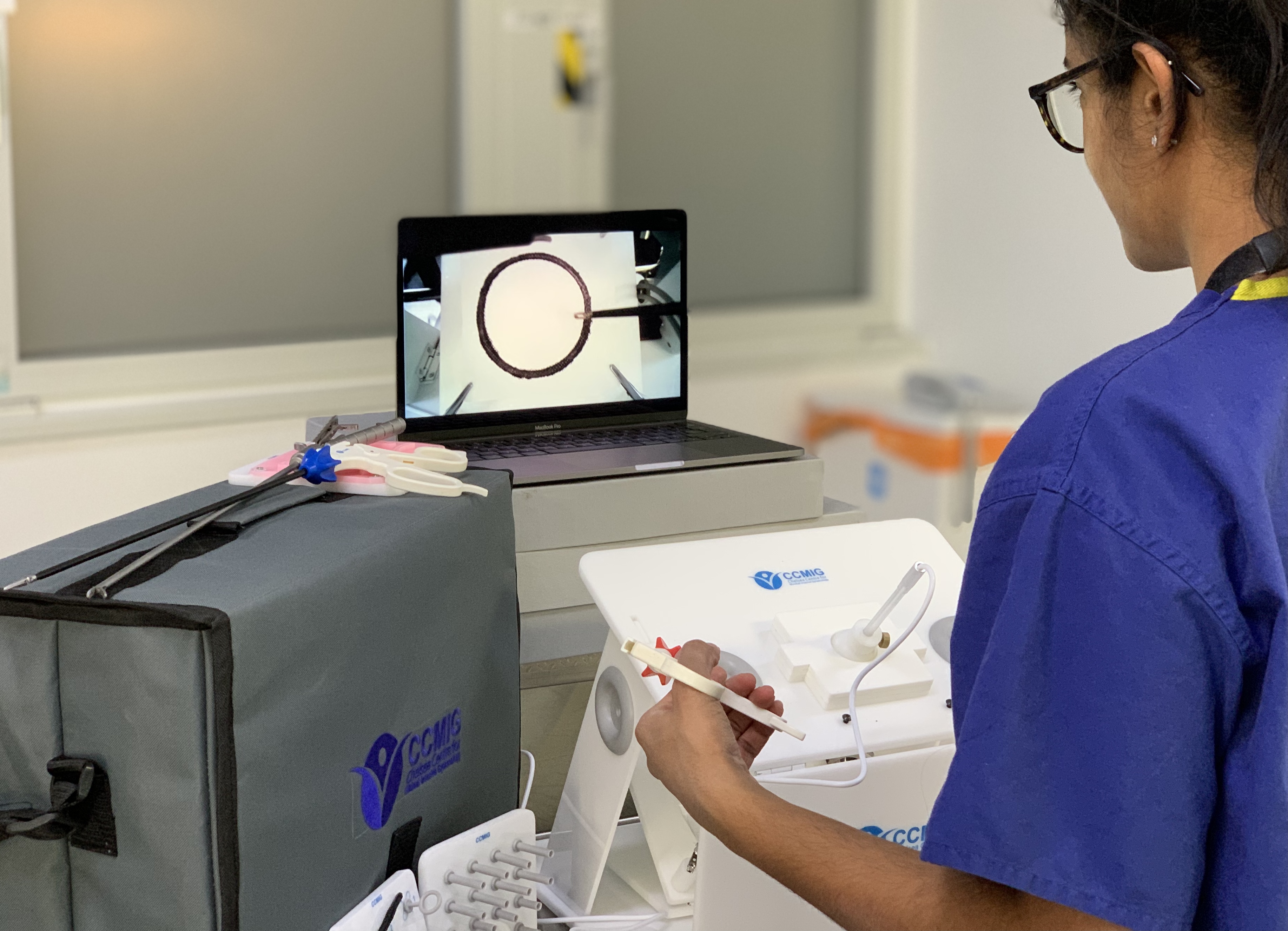
CCMIG Online Course
By CCMIG
Online modular laparoscopy course with theory and practical training. Assignments submitted for each module for progression on to the next.
Medical Receptionist and Medical Terminology Training - Double Endorsed Certificate
By Imperial Academy
2 QLS Endorsed Course | CPD Certified | Free PDF + Hardcopy Certificates | 80 CPD Points | Lifetime Access

Immediate Management of Asthma (e-Learning)
By Guardian Angels Training
Gain essential knowledge and practical skills in managing acute asthma exacerbations with our comprehensive e-learning course. Designed for healthcare professionals, first responders, students, caregivers, and individuals interested in medical care. Enroll now!
Immediate Management of Anaphylaxis (e-Learning)
By Guardian Angels Training
Gain essential knowledge and practical skills related to anaphylaxis with our comprehensive e-learning course. Designed for healthcare professionals, first responders, students, caregivers, and individuals interested in medical care.