- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
332 Medicine & Nursing courses in Grantham delivered Live Online
Clinical coach training for non- Abbeydale Training Practices- May 2025 cohort
By Samantha Morgan-Hourd
This is an online course sat at your own pace to introduce you to clinical coaching. During the course we will go into the concept of training a student, learning methods, tutorial techniques, safeguarding and how to use the Central Skills Log (CSL). The course is open for 2 months. Once completed we can then arrange access to the CSL for one of Abbeydale’s students.

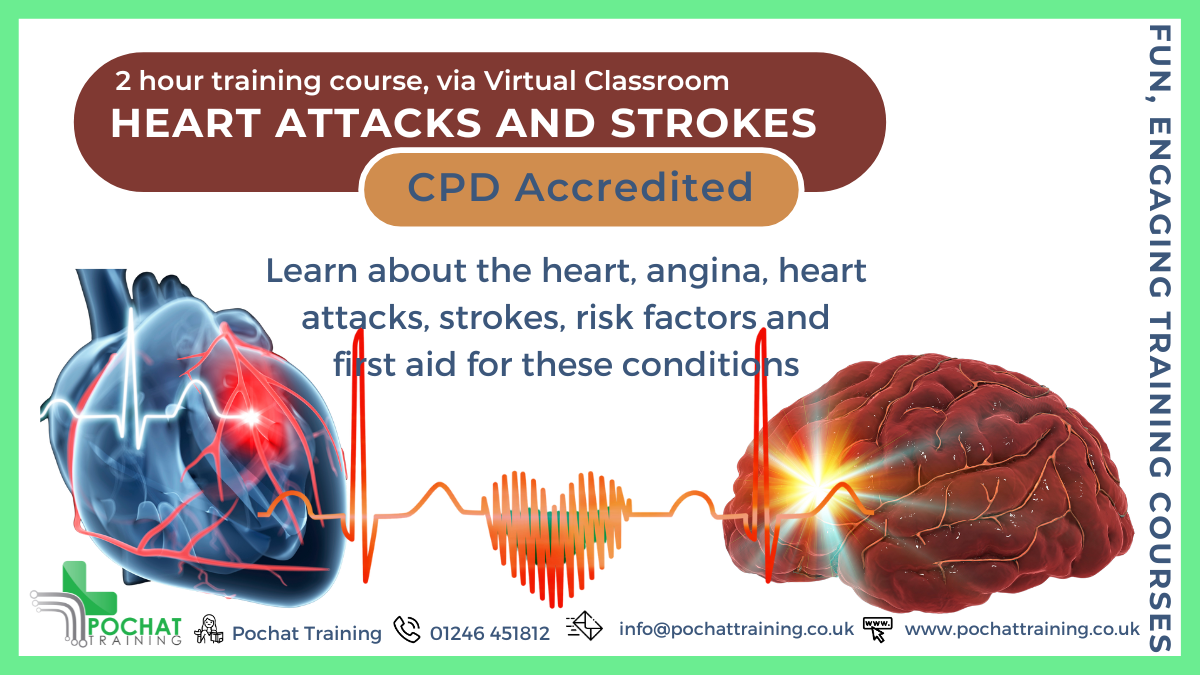
CPD Accredited, Interactive Short Course 2 hr session Do you know what a heart attack or a stroke really is? Would you know what to do to ensure the best possible outcome? Course Contents: How the Heart Functions (overview) Coronary Arteries and Collateral Circulation Atherosclerosis Angina Pectoris Myocardial Infarction (Heart Attack) Treatments and First Aid Treatments Stroke Haemorraghic Stroke, Ischaemic Stroke, Transient Iscaemic Attacks Signs & Symptoms of strokes First Aid Treatment Risk Factors for Angina, Heart Attacks and Strokes Benefits of this Short Course: Learning from home is a good way to keep one's skills and knowledge up to date Over 900,000 people in the UK live with heart failure In the UK, there are more than 100,000 hospital admissions each year due to heart attacks There are also more than 100,000 strokes in the UK each year Would you know what to do to save their life? You can make a huge difference, so join our webinar to find out how
Level 4 Dental Practice Management
By Cavity Dental Training
Unlock Your Potential as a Dental Practice Manager with Our Level 4 Course The Level 4 Diploma in Dental Practice Management qualification delivered by Cavity Training has been designed as a standalone qualification or as an apprenticeship qualification for anyone wishing to become a qualified practice manager. Candidates are required to be in a work placement in order to be able to commence the qualification. Learn about the Cavity Training Dental Practice Management course This qualification allows candidates to learn, develop and practice the skills required for employment and a career in Dental Practice Management. The content covers all essential areas of Management and is mapped to the GDC Learning Outcomes for Dental Nurses and relevant National Occupational Standards. This qualification is approved by the GDC. This qualification allows candidates to go on to higher level 5 management courses and expand their management career. Fees This course can be completed as an government funded apprenticeship, through Cavity Training, or as a privately funded course for £3500. You can either pay as a lump sum or alternatively, you can split into 12 instalments. Entry requirements The minimum requirements are level 2 in English and Maths. Structure To achieve the Level 4 Diploma in Dental Practice Management, candidates will need to also successfully complete a final online Knowledge Test. This is a 24 month course, with a final end point assessment. Knowledge Our course is delivered via live training webinars with specialist tutors. Skills and Behaviours You will be appointed a designated Assessor, who will coach you through your qualification and complete regular assessments with you to support you to complete your qualification. You will have weekly contact from your Assessor. How we compare with our competitors? Don't just take our word for it, here is what our staff think Bridget I did my course years ago. It was classroom based one night per week. I think I would prefer to be more ‘hands on’ like it is now. Cavity really are a great company to work for. I truly believe that there expertise will ensure the next generation are amazing! Gina I did mine over an apprenticeship but the company my employer used wasn’t great and I didn’t get much support. Although I passed I can only imagine the length that Cavity have gone to to ensure that the students feel supported. As an employee, its super! Enquire Today

Learn how to perform and read an ECG ... Nationally Recognised Qualification OCN Accredited - Level 3 (advanced level) CPD Accredited - The CPD Certification Service Introduces you to the fundamentals of setting up and operating an ECG machine Includes patient preparation Produce a valid (error free) ECG Learn and understand ECG traces Recognise recordings that require urgent attention Basic understanding of English language required OPEN TO ALL APPLICANTS VIRTUAL CLASSROOM OPTION INCLUDES COMPREHENSIVE PRACTISE@HOME ECG TRAINING KIT Final interpretation of all ECG recordings is the responsibility of a medical professional.
Level 3 Diploma in Dental Nursing
By Cavity Dental Training
Crafting Dental Excellence: A Comprehensive Journey Backed by City and Guilds Kickstart your transformative journey in dental care with Cavity Training’s Level 3 Diploma in Dental Nursing. Endorsed by City and Guilds, this 11-unit course is meticulously curated by industry stalwarts, providing a harmonious fusion of in-depth theoretical knowledge and tangible hands-on experience. Spanning 18-24 months, the programme immerses you in our interactive virtual classroom, punctuated with in-workplace assessments and culminating in a final exam. Whether you’re charting a new career path or seeking to amplify your stature in dental healthcare, our diploma offers a robust foundation, empowering you to flourish in any dental setting. Learn about the Cavity Training Dental Nurse Course Here at Cavity we have built a reputation on trust and reliability. In recent years the industry needs have shifted and we have had to think of more ways to help our much-loved clients. With huge deficits in the nursing field we have developed a course in partnership with City and Guilds which surpasses all the criteria needed to ensure a fulfilling career in Dental Nursing. One Course Fee The cost is £2500. You can either pay as a lump sum or alternatively, you can split into 12 installments of £209. One to One Contact As well as an online e-portfolio, each student will have a designated assessor assigned to them who will support them through the course as well as in-surgery visits. Full E-library included Exclusively to Cavity Training we have the full e-Library of over 40 textbooks with all the resources you need for your coursework. BADN Membership FREE student membership to The British Association of Dental Nurses (BADN) once you start your training with us. 100+ Years Experience We have 33 assessors and tutors across the country with a vast amount of experience between them. Backed by City & Guilds Cavity Training has worked hard to gain accreditation from City and Guilds and are proud to be working with them. How we compare with our competitors? Don't just take our word for it, here is what our staff think Bridget I did my course years ago. It was classroom based one night per week. I think I would prefer to be more ‘hands on’ like it is now. Cavity really are a great company to work for. I truly believe that there expertise will ensure the next generation are amazing! Gina I did mine over an apprenticeship but the company my employer used wasn’t great and I didn’t get much support. Although I passed I can only imagine the length that Cavity have gone to to ensure that the students feel supported. As an employee, its super! Enquire Today

Auditing Computerised Systems
By Research Quality Association
Course Information Join our comprehensive three-day programme designed as an invaluable external training opportunity for auditors, audit programme managers, and individuals subject to audits. This course is tailored to foster a deep understanding and cultivate essential skills for auditing the validation of computer systems intended for GxP environments (GLP, GCP, GMP, GDP, GPvP). Commencing with an overview of regulatory prerequisites and the system life cycle, the course swiftly transitions to focus on the pragmatic aspects of auditing computer system validation. Experience a blend of presentations, interactive discussions, and immersive practical workshops throughout the duration of the course. Delegates will benefit from practical examples of how to understand the framework of applicable regulations and guidance. Apply risk management techniques to audit planning Plan and conduct computerised system audits Assess system validation documentation to verify compliance Evaluate data integrity and security issues Prepare for regulatory inspection. The course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Understand the vulnerabilities of computerised systems Learn how to create a compliance checklist Link system development with good business practice. Is this course for you? Auditors Audit programme managers Individuals subject to audits. Tutors Tutors will be comprised of (click the photos for biographies): Nichola Stevens Director and Principal Consultant, Nuncius Compliance Solutions Ltd Barry McManus Consultancy Partner, Empowerment Quality Engineering Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Welcome and Introductions 09:45 Why We Validate and Regulatory Trends 10:30 Break 10:45 Audit Overview, High Level Process and Scheduling 11:30 System Lifecycle 12:30 Lunch 13:15 Exercise 1 - Audit Scheduling 14:45 Exercise 1 - Feedback 15:15 Break 15:30 Validation Deliverables 16:30 Risk Assessments 17:30 Close of Day 1 Day 2 09:00 Supplier Assessment 10:30 Break 10:45 Exercise 2 - Planning a Supplier Audit 12:00 Exercise 2 - Feedback 12:30 Lunch 13:15 Exercise 3 - Auditing a Computerised System Validation Package 15:30 Break 15:45 Exercise 3 - Feedback 16:30 Change Control 17:15 Close of Day Day 3 09:00 Infrastructure Qualification 09:45 Maintaining a Validated State - Operational Processes 11:00 Break 11:15 Exercise 4 - Auditing Systems in Operational Use 12:45 Lunch 13:30 Exercise 4 - Feedback 14:15 Exercise 5 - Auditing Trail Review 15:30 Break 15:45 Exercise 5 - Feedback 16:15 Course Objectives Summary and Any Additional Questions 16:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 19 Points Development Level Develop

Access to HE Diploma (Healthcare Professions)
By Egraduate College
Access to Nursing and Healthcare Professions. Regulated by QAA. Widely accepted by UK universities.

Access to HE Diploma (Medicine)
By Egraduate College
Access to Medicine. Regulated by QAA. Widely accepted by UK universities.

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

