- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
771 Manufacturing courses delivered Online
Fundamentals of Mechanical Engineering Mini Bundle
By Compete High
The Fundamentals of Mechanical Engineering Mini Bundle is built for job-seekers who want to work hands-on with real systems—whether in industrial settings, transportation, or tech-driven environments. With a carefully curated skill set in mechanical engineering, basic electrical engineering, physics, bike maintenance, and purchasing and procurement, this bundle makes you immediately more employable in a variety of high-demand fields. Don’t miss your chance to stand out—skilled candidates are getting snapped up fast. Description This bundle is all about building a hireable foundation. If you're aiming for mechanical trades, repair and servicing, supply chain coordination, or hands-on technical roles, mastering mechanical engineering, basic electrical engineering, physics, bike maintenance, and purchasing and procurement sets you apart. Start with mechanical engineering—the backbone of industries like automotive, manufacturing, HVAC, and machinery servicing. Employers consistently look for people who understand mechanical engineering systems and principles. Combine that with basic electrical engineering, and you unlock cross-functional roles that demand both mechanical and electrical fluency. The inclusion of physics further broadens your job-readiness. Whether calculating loads, forces, or motion, physics understanding is vital in mechanical environments. Employers appreciate candidates who grasp real-world physics applications, especially when tied to mechanical systems. Add to that the practical, job-ready nature of bike maintenance. As green transport rises, bike maintenance is gaining relevance fast—in cycling shops, delivery services, fleet maintenance, and public programs. Knowing bike maintenance gives you an immediate advantage in hands-on, trade-based roles. Finally, purchasing and procurement rounds out the bundle by adding operational value. Employers are hiring people who understand sourcing, cost-control, and supply chains. In technical fields, someone who knows purchasing and procurement is often the most valuable hire. From garages and repair shops to supply chain offices and service departments, mechanical engineering, basic electrical engineering, physics, bike maintenance, and purchasing and procurement are exactly what employers want right now. FAQ Q: Who should enrol in this bundle? Anyone seeking a role in mechanical engineering, basic electrical engineering, physics, bike maintenance, or purchasing and procurement—especially those entering technical or industrial careers. Q: Will it help me get a job? Absolutely. This bundle delivers exactly what employers are hiring for: a strong grounding in mechanical engineering, basic electrical engineering, physics, bike maintenance, and purchasing and procurement. Q: What job roles does it apply to? Technical support, servicing and repair, supply chain coordination, entry-level mechanical engineering roles, and bike tech positions—all benefit from these combined skills. Q: Why should I start now? Opportunities are growing fast. Get qualified in mechanical engineering, basic electrical engineering, physics, bike maintenance, and purchasing and procurement before the hiring surge passes you by.
The Production Manager Course helps you learn how to lead teams and manage production smoothly. It gives you a clear understanding of how to guide a group, solve problems, and make decisions. You will learn how to deal with people, build teamwork, and handle tough situations in a smart way. This course shows how to lay the groundwork for successful team management. It covers key models like Tuckman and Jensen’s team development stages and teaches you how to reach group decisions, handle group conflict, and apply helpful techniques to keep things running well. Course Curriculum ✓ Module One: Understanding Facilitation ✓ Module Two: Process vs. Content ✓ Module Three: Laying the Groundwork ✓ Module Four: Tuckman and Jensen’s Model of Team Development ✓ Module Five: Building Consensus ✓ Module Six: Reaching a Decision Point ✓ Module Seven: Dealing with Difficult People ✓ Module Eight: Addressing Group Dysfunction ✓ Module Nine: About Intervention ✓ Homemade Pet Food – Why it is so beneficial Module Ten: Intervention Techniques Learning Outcomes Understand what facilitation means in a team setting. Learn the difference between process and content. Know how to prepare for successful team meetings. Use the Tuckman and Jensen model to understand team growth. Build agreement and encourage group support. Guide your team to make clear decisions. Handle difficult people in a fair and firm way. Spot signs of group issues and manage them. Learn when and how to step in as a manager. Use simple intervention tools to improve teamwork. Who is this course for? This course is for anyone who manages people or leads teams. It’s great for current or future production managers, team leaders, or supervisors in any industry. If you want to boost your leadership and group management skills, this course is for you. Eligibility Requirements You don’t need any formal training to take this course. Anyone with a basic interest in leadership or production can join. A little experience with team settings may help, but it’s not required. Career Path After completing this course, you can explore roles in production, manufacturing, operations, and team leadership. It supports job growth in industries like retail, construction, logistics, or engineering. This course builds the skills you need to lead, manage teams, and move into higher roles. (Learn more about this online course)

Step into the evolving world of design with our CAD Technician Assistant Course – a fully online programme designed for those who are eager to understand the foundations of Computer-Aided Design. Whether you're looking to explore CAD from scratch or sharpen your digital design understanding, this course walks you through the essentials of 2D and 3D modelling, geometry, assemblies, extrusion techniques, and more. It also offers an introduction to popular CAD software such as AutoCAD, SolidWorks, T-Flex, and FreeCAD – all without needing to install a single drafting table. Ideal for learners aiming to support design teams or pursue CAD-focused roles, this course covers everything from geometric modelling to civil drawing parameters. It’s tailored with a clear, progressive structure that builds your knowledge step-by-step – from basic shape creation through to the intricacies of blending operations and working with assemblies. You'll gain a strong foundation in design software tools widely used in engineering, manufacturing, and architecture sectors. All modules are structured to suit self-paced learning, and the course is delivered entirely online with flexibility in mind. Key Benefits Accredited by CPD Instant e-certificate Fully online, interactive course Self-paced learning and laptop, tablet, smartphone-friendly 24/7 Learning Assistance Curriculum Module 01: Introduction to CAD Design Module 02: CAD Designer Career in the United Kingdom Module 03: Basics for CAD Design - Part 1 Module 04: Basics for CAD Design - Part 2 Module 05: 2D Shape in CAD Module 06: 3D Shape in CAD Module 07: Geometry and Modelling Module 08: Assemblies in CAD Design Module 09: Extrusion and Rotations Module 10: Blending Operations in CAD Module 11: Grids and Regular Polygons Module 12: Parameters in Civil Drawings Module 13: Introduction to AutoCAD Module 14: Introduction to T-Flex CAD Module 15: Introduction to SolidWorks Module 16: Introduction to FreeCAD Course Assessment You will immediately be given access to a specifically crafted MCQ test upon completing an online module. For each test, the pass mark will be set to 60%. Certificate Once you've successfully completed your course, you will immediately be sent a digital certificate. Also, you can have your printed certificate delivered by post (shipping cost £3.99). Our certifications have no expiry dates, although we do recommend that you renew them every 12 months. CPD 10 CPD hours / points Accredited by CPD Quality Standards Who is this course for? The CAD Technician Assistant training is ideal for highly motivated individuals or teams who want to enhance their skills and efficiently skilled employees. Requirements There are no formal entry requirements for the course, with enrollment open to anyone! Career path Learn the essential skills and knowledge you need to excel in your professional life with the help & guidance from our CAD Technician Assistant training. Certificates Certificate of completion Digital certificate - Included Certificate of completion Hard copy certificate - Included

Think of a warehouse as a giant puzzle, and warehouse management as the art of making every piece fit — quickly, efficiently and without chaos. This Warehouse Management Diploma Accredited Career Bundle Course is your guide to mastering that art. You’ll learn how to organise stock, manage supply chains, and keep things moving like clockwork behind the scenes of busy businesses. Whether it’s planning deliveries or managing inventory, this course walks you through the essentials in a clear and structured way — all from the comfort of your screen. Warehousing isn’t just about storing boxes — it’s about keeping operations smooth, saving costs, and making sure the right item lands in the right hands at the right time. This course bundle covers multiple key areas in warehouse operations, logistics, and inventory systems. If you’re eyeing a role that keeps the backbone of retail, manufacturing or distribution running — or just want to get your foot in the door — this is a smart place to start. No confusing jargon. No unnecessary fluff. Just solid knowledge you can actually use. Job Responsibilities in Warehouse Management: Managing inventory and ensuring accurate stock levels Organising and optimising warehouse layout and storage systems Coordinating and overseeing the shipping and receiving processes Implementing and maintaining efficient logistics and distribution procedures Monitoring and maintaining quality control standards Managing a team of warehouse staff and providing training as needed Liaising with suppliers, vendors, and other departments to ensure smooth operations Skills needed to be successful in Warehouse Management jobs: Strong organisational and time management abilities Excellent problem-solving and decision-making skills Proficient in using warehouse management systems and software Solid knowledge of health and safety regulations and procedures Effective communication and interpersonal skills for team management Attention to detail and accuracy in handling inventory and orders Ability to adapt to changing priorities and work well under pressure Expected Salaries in Warehouse Management (UK): Entry-level positions: £20,000 - £25,000 per annum Mid-level positions: £25,000 - £35,000 per annum Senior-level positions: £35,000 - £50,000+ per annum Key Features of Warehouse Management Career Bundle CPD Accredited Warehouse Management Courses Instant PDF certificate Fully online, interactive course Self-paced learning and laptop, tablet, smartphone-friendly 24/7 Learning Assistance Discounts on bulk purchases Course 01: Warehouse Operative The Warehouse Operative course provides comprehensive training, starting from the basics. It equips you with the knowledge and skills necessary to become a warehouse operative. By successfully completing this course, you can obtain a professional certificate for free, showcasing your accomplishments in the professional field. This course is designed to provide a detailed understanding of the nature of the warehouse industry and your key roles within it. Course 02: Forklift Training The Forklift Training course offers in-depth instruction on forklift operations. It covers everything you need to know, starting from scratch, ensuring you have a solid foundation in forklift handling and safety. This comprehensive course is designed to provide a detailed understanding of the forklift industry and your key responsibilities within it. Course 03: Transport and Logistics Course 03, Transport and Logistics, provides comprehensive training in the field of transportation and logistics. It covers all aspects of the industry, starting from the fundamentals, allowing you to develop a deep understanding of the field. This course is designed to equip you with the necessary knowledge and skills to excel in various roles within the transport and logistics sector. Course 04: Manual Handling Interactive Training Course 04, Manual Handling Interactive Training, is a comprehensive program that covers the topic of manual handling in detail. It starts from scratch, providing thorough guidance on safe lifting and moving techniques to prevent injuries in the workplace. By completing this course, you can achieve a professional certificate at no cost, highlighting your expertise in manual handling and your commitment to workplace safety. Course 05: RIDDOR Training Course 05, RIDDOR Training, is a comprehensive program designed to educate individuals on the Reporting of Injuries, Diseases, and Dangerous Occurrences Regulations (RIDDOR). This course covers the regulations and procedures related to reporting workplace incidents and accidents. Course 06: LOLER Training Course 06, LOLER Training, is specifically tailored for individuals working in roles such as LOLER technicians, construction workers, warehouse operatives, health and safety managers, crane operators, and engineering surveyors. This comprehensive course aims to enhance their skills and knowledge in Lifting Operations and Lifting Equipment Regulations (LOLER). Course 07: Ladder Safety Course 07, Ladder Safety, offers comprehensive training on ladder usage and safety precautions. Starting from the basics, this course covers important aspects such as ladder selection, inspection, setup, and safe climbing techniques. Course 08: Supply Chain Management Course 08, Supply Chain Management, provides a comprehensive understanding of the principles and practices involved in managing the flow of goods and services in a supply chain. This course covers various aspects, including procurement, production, inventory management, and logistics. Course 09: Procurement and Supply Chain Management Course 09, Procurement and Supply Chain Management offers in-depth training on the procurement process and its integration within the broader supply chain framework. It covers topics such as sourcing, supplier management, contract negotiation, and risk assessment. Course 10: Advanced Health and Safety Course 10, Advanced Health and Safety, is a comprehensive program that delves into various aspects of workplace health and safety management. Starting from scratch, this course covers advanced topics, including risk assessment, hazard identification, emergency preparedness, and regulatory compliance. Accreditation This Warehouse Management bundle courses are CPD accredited, providing you with up-to-date skills and knowledge and helping you to become more competent and effective in your chosen field. Certification Once you've successfully completed your Warehouse Management course, you will immediately be sent a digital certificate. Also, you can have your printed certificate delivered by post (shipping cost £3.99). CPD 100 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone with a knack for learning new skills can take this Warehouse Management Diploma course. While this comprehensive training is popular for preparing people for job opportunities in the relevant fields, it also helps to advance your career for promotions. Requirements No prior degree or experience is required to enrol in this course. Career path This Warehouse Management Course will help you to explore avariety of career paths in the related industry, including: Warehouse Manager Logistics Coordinator Inventory Controller Distribution Supervisor Warehouse Operations Manager Supply Chain Analyst Procurement Officer Certificates Digital certificate Digital certificate - Included Hardcopy Certificate Hard copy certificate - Included Hardcopy Certificate (UK Delivery): For those who wish to have a physical token of their achievement, we offer a high-quality, printed certificate. This hardcopy certificate is also provided free of charge. However, please note that delivery fees apply. If your shipping address is within the United Kingdom, the delivery fee will be only £3.99. Hardcopy Certificate (International Delivery): For all international addresses outside of the United Kingdom, the delivery fee for a hardcopy certificate will be only £10.

Soap Making Techniques: Craft Attractive & Natural Soaps at Home
By Wise Campus
Soap Making: Soap Making Course Online Are you prepared to embark on a path of indulging in lavish self-care, self-expression, and creativity? Look nowhere else! Your soap-making abilities will soar to new heights with the help of our handmade soap making course. During this handmade soap making course, you will learn the methods for manufacturing soap that have been passed down through the years. The handmade soap making course covers a broad range of subjects, from fundamental methods to more complex ones. Also, you will have the opportunity to experiment with various soup components in the handmade soap making course. To learn more about the world of handmade soap making, enrol in our special Handmade Soap Making course right away! Main Course: Soap Making Course Free Courses are including with this Soap Making: Soap Making Course. Along with The Soap Making: Soap Making Course, We Offer a free Dress Making and Fashion Design Course Special Offers of this Soap Making: Soap Making Course This Soap Making: Soap Making Course includes a FREE PDF Certificate. Lifetime access to this Soap Making: Soap Making Course Instant access to this Soap Making: Soap Making Course Get FREE Tutor Support to this Soap Making: Soap Making Course Soap Making: Soap Making Course Online This Soap Making: Handmade Soap Making Course will teach you the trade secrets for creating gorgeous, nourishing soaps with premium ingredients. Beginners may find our Soap Making: Handmade Soap Making Course ideal as it begins from scratch. Discover the essential equipment, safety precautions, and basic soap-making methods with this course on handmade soap production Who is this course for? Soap Making: Soap Making Course Online Both experienced soap makers and those who want to know more about the process will benefit from this handmade soap making course. Requirements Soap Making: Soap Making Course Online To enrol in this Soap Making: Soap Making Course, students must fulfil the following requirements. To join in our Soap Making: Soap Making Course, you must have a strong command of the English language. To successfully complete our Soap Making: Soap Making Course, you must be vivacious and self driven. To complete our Soap Making: Soap Making Course, you must have a basic understanding of computers. A minimum age limit of 15 is required to enrol in this Soap Making: Soap Making Course. Career path Soap Making: Soap Making Course Online You can flourish in the appropriate field by completing this handmade soap making course, which will assist you in gaining all the necessary theoretical knowledge.

Food Allergen: Food Allergen Course Online Unlock the Power of Food Allergen: Food Allergen Course: Enrol Now! Do you work for a company that makes, sells, or serves food? Are you interested in working in the hospitality, food manufacturing, or retail industries? Then you must take this Food Allergen: Food Allergen Course. This Food Allergen: Food Allergen Course is essential for almost everyone, as it is becoming increasingly important that we all understand food allergens and their effects on people. Food Allergen: Food Allergen Course are caused by allergens, which are proteins found in food. If allergic reactions are not properly identified, managed, and treated, they can be severe and even life-threatening. As the number of people affected by food allergies increases, so does awareness of allergens around the world. As a result, it is critical to stay informed and prepare an action plan in case of a reaction. Industry professionals design the Food Allergen: Food Allergen Course to help you gain knowledge and skills with ease. So, enrol today and start learning! Main Course: Food Allergen Awareness Training Free Courses included with Food Allergen: Food Allergen Course Along with Food Allergen Course you will get free Level 2 Food Hygiene and Safety Along with Food Allergen Course you will get free Level 2 HACCP Training Course For Catering & Retail Along with Food Allergen Course you will get free Level 2 Award in the Prevention and Control of Infection Special Offers of this Food Allergen: Food Allergen Course: This Food Allergen: Food Allergen Course includes a FREE PDF Certificate. Lifetime access to this Food Allergen: Food Allergen Course Instant access to this Sales Food Allergen: Food Allergen Course 24/7 Support Available to this Food Allergen: Food Allergen Course Food Allergen: Food Allergen Course Online This Food Allergen: Food Allergen Course will introduce food allergens to raise awareness and increase knowledge about the topic. We will also look at how food allergies affect the body to boost confidence and reduce the risks associated with allergic reactions. Who is this course for? Food Allergen: Food Allergen Course Online Anyone who works with food should take this Food Allergen: Food Allergen Course. Requirements Food Allergen: Food Allergen Course Online To enrol in this Food Allergen: Food Allergen Course, students must fulfil the following requirements: Good Command over English language is mandatory to enrol in our Food Allergen: Food Allergen Course. Be energetic and self-motivated to complete our Food Allergen: Food Allergen Course. Basic computer Skill is required to complete our Food Allergen: Food Allergen Course. If you want to enrol in our Food Allergen: Food Allergen Course, you must be at least 15 years old. Career path Food Allergen: Food Allergen Course Online Obtaining certification and acquiring skills through Food Allergen Awareness can significantly enhance your professional prospects, enabling you to excel in various domains. This expertise opens doors to lucrative opportunities within sectors associated with Food Allergen Awareness, positioning you to pursue high-paying careers in related fields.

Food Allergen: Food Allergen Course Online Unlock the Power of Food Allergen: Food Allergen Course: Enrol Now! Do you work for a company that makes, sells, or serves food? Are you interested in working in the hospitality, food manufacturing, or retail industries? Then you must take this Food Allergen: Food Allergen Course. This Food Allergen: Food Allergen Course is essential for almost everyone, as it is becoming increasingly important that we all understand food allergens and their effects on people. Food Allergen: Food Allergen Course are caused by allergens, which are proteins found in food. If allergic reactions are not properly identified, managed, and treated, they can be severe and even life-threatening. As the number of people affected by food allergies increases, so does awareness of allergens around the world. As a result, it is critical to stay informed and prepare an action plan in case of a reaction. Industry professionals design the Food Allergen: Food Allergen Course to help you gain knowledge and skills with ease. So, enrol today and start learning! Main Course: Food Allergen Awareness Training Free Courses included with Food Allergen: Food Allergen Course Along with Food Allergen Course you will get free Level 2 Food Hygiene and Safety Along with Food Allergen Course you will get free Level 2 HACCP Training Course For Catering & Retail Along with Food Allergen Course you will get free Level 2 Award in the Prevention and Control of Infection Special Offers of this Food Allergen: Food Allergen Course: This Food Allergen: Food Allergen Course includes a FREE PDF Certificate. Lifetime access to this Food Allergen: Food Allergen Course Instant access to this Sales Food Allergen: Food Allergen Course 24/7 Support Available to this Food Allergen: Food Allergen Course Food Allergen: Food Allergen Course Online This Food Allergen: Food Allergen Course will introduce food allergens to raise awareness and increase knowledge about the topic. We will also look at how food allergies affect the body to boost confidence and reduce the risks associated with allergic reactions. Who is this course for? Food Allergen: Food Allergen Course Online Anyone who works with food should take this Food Allergen: Food Allergen Course. Requirements Food Allergen: Food Allergen Course Online To enrol in this Food Allergen: Food Allergen Course, students must fulfil the following requirements: Good Command over English language is mandatory to enrol in our Food Allergen: Food Allergen Course. Be energetic and self-motivated to complete our Food Allergen: Food Allergen Course. Basic computer Skill is required to complete our Food Allergen: Food Allergen Course. If you want to enrol in our Food Allergen: Food Allergen Course, you must be at least 15 years old. Career path Food Allergen: Food Allergen Course Online Obtaining certification and acquiring skills through Food Allergen Awareness can significantly enhance your professional prospects, enabling you to excel in various domains. This expertise opens doors to lucrative opportunities within sectors associated with Food Allergen Awareness, positioning you to pursue high-paying careers in related fields.

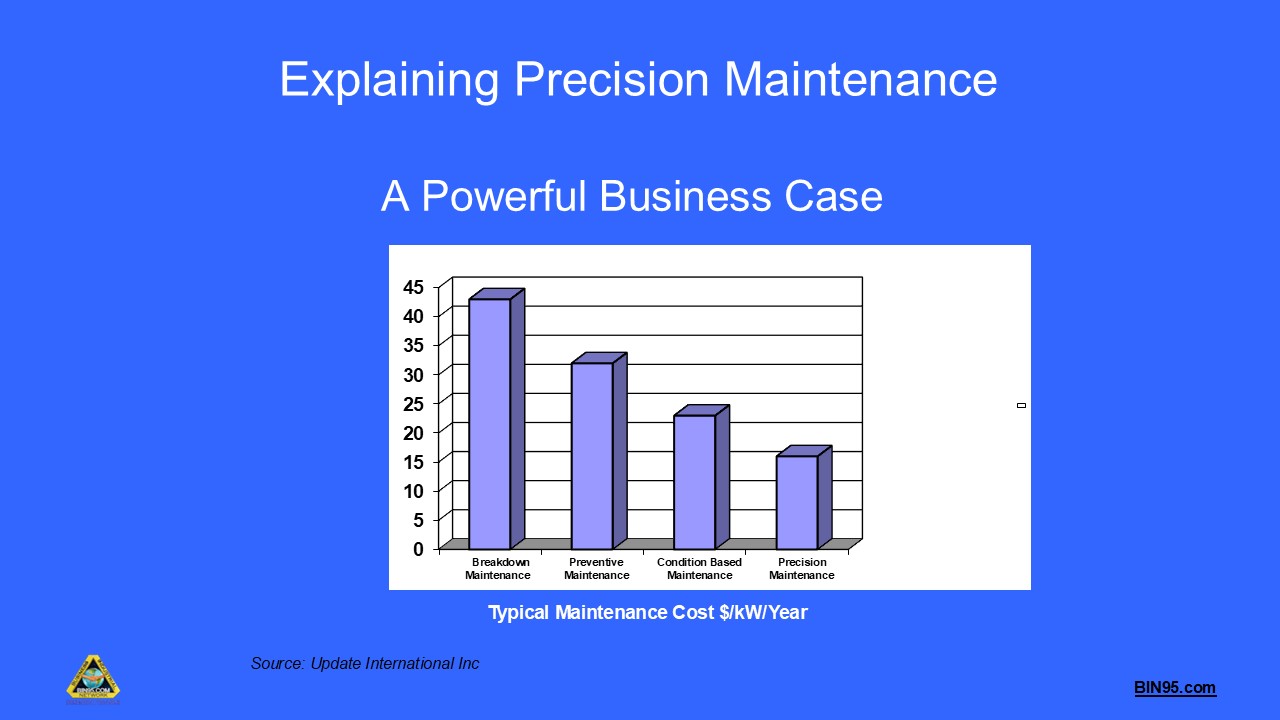
This free course, provided by the BIN95 Manufacturing Training Division, provides a comprehensive understanding of precision maintenance as a critical component of world-class rotating machinery reliability. Participants will learn the business case for precision maintenance, the standards and best practices required, and the practical steps to implement precision maintenance in industrial environments. The course covers key topics, including vibration analysis, alignment, balancing, lubrication, fit and tolerance, torque standards, and the development of a precision maintenance culture. Through real-world examples, standards references (ISO, ANSI), and practical procedures, learners will gain the skills to reduce maintenance costs, increase equipment reliability, and drive continuous improvement in plant operations. Ideal for maintenance professionals, engineers, and reliability managers seeking to elevate their maintenance practices to world-class standards.
Mechanical Engineering
By Wise Campus
Mechanical Engineering: Mechanical Engineering Do you want to gain an understanding of core principles in mechanics? Our mechanical engineering course will help you to do so. This mechanical engineering course explains thermodynamics and materials science. This mechanical engineering course teaches how to design, analyse, and optimise mechanical systems and components. The mechanical engineering course topics include fluid dynamics, heat transfer, and machine design. Moreover, the mechanical engineering course describes control systems, CAD modelling, prototyping, and testing methodologies. The mechanical engineering course emphasises sustainability, innovation, and interdisciplinary collaboration to address contemporary engineering challenges. Participate in the mechanical engineering course to contribute to the development of cutting-edge technologies and solutions for global advancement. Learning outcome of mechanical engineering course The mechanical engineering course will provide idea of: Introduction to mechanical engineering, engineering mathematics and physics fundamental. Mechanical engineering course explains materials science, thermodynamics and heat transfer. Fluid mechanics and aerodynamics are describes in this mechanical engineering course. Moreover, mechanics of solids, structural analysis, machine design and kinematics are parts of this mechanical engineering course. You will understand about the dynamics, control systems and capstone design projects with this mechanical engineering course. Mechanical engineering course teaches manufacturing processes, technology, sustainability and renewable energy process. After the mechanical engineering course completion you will learn experimental methods, proper data analysis, professional development and engineering ethics. Special Offers Of this Mechanical Engineering: Mechanical Engineering Course This Mechanical Engineering: Mechanical Engineering Course includes a FREE PDF Certificate. Lifetime access to this Mechanical Engineering: Mechanical Engineering Course Instant access to this Mechanical Engineering: Mechanical Engineering Course Get FREE Tutor Support to this Mechanical Engineering: Mechanical Engineering Course Mechanical Engineering: Mechanical Engineering Gain a deep understanding of core principles with our comprehensive Mechanical Engineering course. Learn about thermodynamics, materials science, and fluid dynamics. Master the design, analysis, and optimization of mechanical systems, covering topics like heat transfer, machine design, and control systems. Our course includes CAD modeling, prototyping, and sustainable engineering practices. Emphasizing innovation and interdisciplinary collaboration, this course prepares you to tackle contemporary engineering challenges. Develop the skills needed for creating advanced technologies and solutions that drive global progress. Enroll now to become a leader in mechanical engineering! Who is this course for? Mechanical Engineering: Mechanical Engineering The mechanical engineering course offers the foundational information required to pursue a career in the field or advance your current understanding. Requirements Mechanical Engineering: Mechanical Engineering To enrol in this Mechanical Engineering: Mechanical Engineering Course, students must fulfil the following requirements. To join in our Mechanical Engineering: Mechanical Engineering Course, you must have a strong command of the English language. To successfully complete our Mechanical Engineering: Mechanical Engineering Course, you must be vivacious and self driven. To complete our Mechanical Engineering: Mechanical Engineering Course, you must have a basic understanding of computers. Career path Mechanical Engineering: Mechanical Engineering In a variety of professional contexts, the mechanical engineering knowledge acquired from certified mechanical engineering course is priceless.

Food Allergen Awareness Training
By Wise Campus
Food Allergen: Food Allergen Awareness Training Live a allergy free life - Enrol in our Food Allergen: Food Allergen Awareness Training to find your greatest passion in living and working for others and for yourself! Are you employed by a business that produces, sells, or provides food? Are you thinking about a career in the hotel, food production, or retail sectors? If yes, you should take this Food Allergen Awareness Training as soon as possible. This Food Allergen Awareness Training is a perfect course for all to comprehend food allergens and how they affect individuals. This Food Allergen Awareness Training Course will introduce food allergens to raise awareness and increase knowledge about the topic. The six separate modules in this food allergen awareness training describe the effects of allergies to various foods. The Food Labelling Rules and Food Allergen Legislation are discussed in the Food Allergen: Food Allergen Awareness Training course.. Moreover, Avoiding Allergenic Cross Contamination is covered in this Food Allergen: Food Allergen Awareness Training course on food allergen awareness. Enrol today to begin learning! You may easily learn new information and skills with the aid of a food allergen awareness training course. Main Course: Food Allergen Awareness Special Offers of this Food Allergen: Food Allergen Training Course This Food Allergen: Food Allergen Course includes a FREE PDF Certificate. Lifetime access to this Food Allergen: Food Allergen Course Instant access to this Food Allergen: Food Allergen Course Get FREE Tutor Support to this Food Allergen: Food Allergen Course Food Allergen: Food Allergen Awareness Training Online Are you employed by a company that produces, distributes, or serves food? Are you considering a career in retail, food manufacturing, or hospitality? Then this Food Allergen: Food Allergen Course is required of you. Nearly everyone should take this Food Allergen: Food Allergen Course since it is crucial that we all comprehend food allergies and how they affect individuals. Who is this course for? Food Allergen Awareness Training The Food Allergen: Food Allergen Training is intended for food handlers and other catering sector employees who are involved in food preparation and serving. Requirements Food Allergen Awareness Training To enrol in this Food Allergen Course, students must fulfil the following requirements. To join in our Food Allergen: Food Allergen Training Course, you must have a strong command of the English language. To successfully complete our Food Allergen: Food Allergen Training Course, you must be vivacious and self driven. To complete our Food Allergen: Food Allergen Training Course, you must have a basic understanding of computers. A minimum age limit of 15 is required to enrol in this Food Allergen: Food Allergen Training Course. Career path Food Allergen Awareness Training The Food Allergen: Food Allergen Training will provide opportunities for a variety of jobs, including: Auditor of food safety: £25,000 to £45,000 annually Coordinator of Quality Assurance: £20,000 to £35,000 annually Expert in Allergen Control: £22,000 to £40,000 annually
