- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
An Understanding of Diabetes, PoC Blood Glucose Monitoring, and the Administration of Insulin
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for effective diabetes management with our course on blood glucose monitoring and insulin administration. Ideal for healthcare professionals.

Overview To achieve maximum effectiveness, managers and professionals must continually expand their business knowledge and sharpen their skills. This program is designed to achieve this goal in a time-efficient manner. Integration of Downstream Refining and Petrochemicals to achieve greater efficiencies is yet another critical factor in this business which will be covered in this course

Global Project Management
By IIL Europe Ltd
Global Project Management In this course, you will dig deeper-and differently-into project management processes, tools, and techniques, developing the ability to see them through the lens of global and cultural project impacts. In today's increasingly global environment, managing a project with customers and support organizations spread across multiple countries and continents is a major challenge. From identifying stakeholders and gathering requirements, to planning, controlling, and executing the project, the basic logistics of a global project present their own standard challenges. However, with additional cultural, language-based, and regional elements, global projects involve more complexities than teams often realize. There are unique communication needs, cultural awareness elements, varying customs and work expectations, and critical legal differences to consider. In this course, you will dig deeper-and differently-into project management processes, tools, and techniques, developing the ability to see them through the lens of global and cultural project impacts. This will leverage you to problem solve differently on global projects, prevent problems, and ensure success. The goal is for you to effectively navigate the challenges of leading projects with multi-regional footprints and globally diverse sets of stakeholders. What you Will Learn At the end of this program, you will be able to: Determine when a project meets the criteria of being a true global one Articulate global project needs based on the project grid and framework Identify and analyze global project stakeholders Recognize cultural differences and articulate how they impact project work Determine global project estimating, scheduling, and staffing challenges Assess global project risks and develop problem-solving responses Analyze complex cultural situations and align optimal project communication and negotiation tools and techniques Apply best practices for conducting virtual team work and mitigating virtual challenges Evaluate ways to control for global project scope, cost, and procurement Align customer management best practices with global customer needs Implement key global project closing activities Foundation Concepts What is a global project? What makes a global project different? A global project management framework Initiating the Global Project Launching a global project Respecting cultural differences Identifying and analyzing stakeholders Developing the communications plan Defining the ideal global project manager Crafting a global project charter Planning the Global Project Gathering requirements for a global project Defining the scope, region by region Estimating and scheduling for global projects Staffing the global project Developing the global risk management plan Executing the Global Project Managing global stakeholder expectations Embracing cultural diversity Honing global negotiation techniques Procuring goods and services on a global basis Managing global legal and regulatory issues at the micro and macro level Monitoring and Controlling the Global Project Status reporting Virtual communication Cost control Schedule control Scope control Customer satisfaction Closing the Global Project Contract closure at the macro and micro levels Administrative closure with global reach Lessons learned

Global Project Management: In-House Training
By IIL Europe Ltd
Global Project Management: In-House Training: In-House Training In this course, you will dig deeper-and differently-into project management processes, tools, and techniques, developing the ability to see them through the lens of global and cultural project impacts. In today's increasingly global environment, managing a project with customers and support organizations spread across multiple countries and continents is a major challenge. From identifying stakeholders and gathering requirements, to planning, controlling, and executing the project, the basic logistics of a global project present their own standard challenges. However, with additional cultural, language-based, and regional elements, global projects involve more complexities than teams often realize. There are unique communication needs, cultural awareness elements, varying customs and work expectations, and critical legal differences to consider. In this course, you will dig deeper-and differently-into project management processes, tools, and techniques, developing the ability to see them through the lens of global and cultural project impacts. This will leverage you to problem solve differently on global projects, prevent problems, and ensure success. The goal is for you to effectively navigate the challenges of leading projects with multi-regional footprints and globally diverse sets of stakeholders. What you Will Learn At the end of this program, you will be able to: Determine when a project meets the criteria of being a true global one Articulate global project needs based on the project grid and framework Identify and analyze global project stakeholders Recognize cultural differences and articulate how they impact project work Determine global project estimating, scheduling, and staffing challenges Assess global project risks and develop problem-solving responses Analyze complex cultural situations and align optimal project communication and negotiation tools and techniques Apply best practices for conducting virtual team work and mitigating virtual challenges Evaluate ways to control for global project scope, cost, and procurement Align customer management best practices with global customer needs Implement key global project closing activities Foundation Concepts What is a global project? What makes a global project different? A global project management framework Initiating the Global Project Launching a global project Respecting cultural differences Identifying and analyzing stakeholders Developing the communications plan Defining the ideal global project manager Crafting a global project charter Planning the Global Project Gathering requirements for a global project Defining the scope, region by region Estimating and scheduling for global projects Staffing the global project Developing the global risk management plan Executing the Global Project Managing global stakeholder expectations Embracing cultural diversity Honing global negotiation techniques Procuring goods and services on a global basis Managing global legal and regulatory issues at the micro and macro level Monitoring and Controlling the Global Project Status reporting Virtual communication Cost control Schedule control Scope control Customer satisfaction Closing the Global Project Contract closure at the macro and micro levels Administrative closure with global reach Lessons learned

If you have at least 5 years working experience and you would like to attain Gold Card status via the Experienced Worker route by joining the City & Guilds 2346 NVQ Level 3, you will also need to hold the below two pre-requisite qualifications: City & Guilds 2391-52 Inspection and Testing Course C&G 2382-22 BS7671 18th Edition

3 Day Asset Management Certificate Course C23010
By Asset Management Consulting (Asset Management Academy)
Global leading Asset Management Certificate course in-person leading to the IAM Certificate and CAMA, Manchester UK, September 2023

Permanent Makeup Course Combinations | Eyes & Lips - Small Group Learning
By ID Liner | Permanent Makeup Training & Supplies
BY MIXING AND MATCHING OUR FUNDAMENTAL BEGINNERS PERMANENT MAKEUP TRAINING COURSES, TRAINEES CAN MAKE SAVINGS OF UP TO £5,145! ALL COURSE COMBINATIONS INCLUDE A FULL PERMANENT MAKEUP KIT.

Overview Internet is the main source of marketing today and Digital Marketing along with Social Media Marketing helps the business to stay alive in the digital world. Earlier companies use to spend a lot of money on traditional ways of marketing and still, they were not able to reach global clients. With the existence of Digital marketing, they are able to break the barriers to reaching global clients. With the use of Social Media Marketing and Digital Marketing organisations are able to reach every corner of the world and also it is very cost-effective. Digital Marketing encompasses various channels like Search Engine Optimisation, Social Media Marketing like usage of Facebook, Facebook marketing, Ad Words, and Email Campaigns and is list is longer. All these tools are used to cover global marketing. No matter where you are located in the world, your product can reach clients anywhere in the world. This course will help develop innovative social media strategies, and to boost brand awareness with rich content. With the effective analysis of campaign results, you'll be on track to exceed sales targets and advance your career with the latest social media marketing techniques.

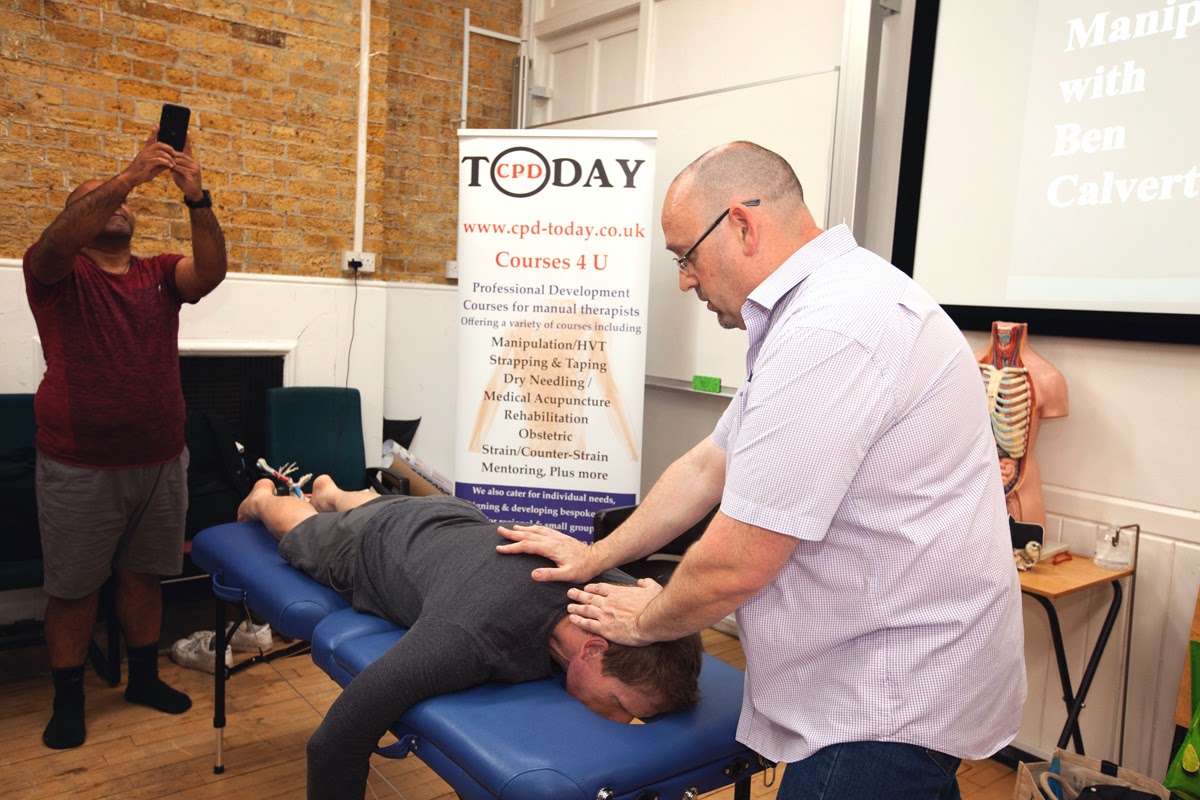
Spinal and Peripheral Manipulation (Grade 5 Mobilisation) Course June 2025
By CPD Today
Spinal and Peripheral Manipulation Grade 5 Mobilisation Course Covering junctional areas and upper and lower extremities. Osteopathy Physiotherapy Chiropractic
Search By Location
- GL Courses in London
- GL Courses in Birmingham
- GL Courses in Glasgow
- GL Courses in Liverpool
- GL Courses in Bristol
- GL Courses in Manchester
- GL Courses in Sheffield
- GL Courses in Leeds
- GL Courses in Edinburgh
- GL Courses in Leicester
- GL Courses in Coventry
- GL Courses in Bradford
- GL Courses in Cardiff
- GL Courses in Belfast
- GL Courses in Nottingham
