- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
CPD Accredited Botox & Dermal Filler Training
By The Angel Academy Of Teaching & Training
FOUR DAY ATTENDENCE AND TRAINING GUIDELINE: DAY ONE AND TWO - INTRODUCTION TO DERMAL FILLERS Day one Arrive and coffees (10.00) Registration, introduction and expectations (10.00-10.15) Structure of the training (10.15-10.30) Break (10.30-11.00) Lectures and interactive workshops / simulation (11.00 - 1230pm) Health and safety in the workplace Sharps injury and disposal The consultation process and prescriptions LUNCH (1300-1730) with a coffee break Basic life support Anaphylaxis - recognition and management Emergency kits - what it should contain and how to buy one Your doctors on call - how to contact our on call doctors for emergency advice How to use Hyalase safely - when to use it / recognise mechanism of action, how prescription in an emergency works and how to give the hyalase Practical and to include demonstration of Hyalase injection Our added benefits services for safety and convenience Day two Arrive and coffees (10.00) introduction and expectations (10.00-10.30) Structure of the training (10.30-11.00) Formal written examination covering key areas of THEORY for Dermal Filler injections: Anatomy, Physiology, Products and Complications. This will highlight early on if any important areas need to be covered in more detail for the students (11.00 - 1200) - Break for lunch - Practical session commences - (12.30 - 1800) - and in total on average we have scope for one model per 30 minutes on both of the Dermal filler days, so that’s a potential for 10 in total for a class size of maximum 4, which will give good hands on experience, as the way we train is to allow several people the opportunity to be involved with each patient - e.g. splitting into the phases of treatment, which allows the trainees to understand the concept of the treatment process. That would be - consultation, consent, marking up, readying equipment, performing the injection, providing advice and aftercare. DAY THREE AND FOUR BOTOX FOUNDATION COURSE Day three Arrive and coffees (10.00) introduction and expectations (10.00 – 10.30) Structure of the training (10.30 – 11.00) Formal written examination covering key areas of THEORY for Botox Application: Anatomy, Physiology, Products and Complications. This will highlight early on if any important areas need to be covered in more detail for the students (11.00 - 1200) - Break for lunch - Practical session commences - (12.30 - 1800) - and in total on average we have one model per 30 minutes on both the botox and days, so that’s a potential of 10 in total for a class size of 4, which will give good hands on experience, as the way we train is to allow several people the opportunity to be involved with each patient - e.g. splitting into the phases of treatment, which allows the trainees to understand the concept of the treatment process. That would be - consultation, consent, marking up, readying equipment, performing the injection, providing advice and aftercare. Day four Observed Treatment Process Examination The participants will be tested on the following key facets of safe practical care: Consultation process - rapport and understanding what the client wants Safe consent Marking and photographs Technical skill of injection Atercare provision and safety netting (eg if this happens do this / call me) 1 model will be provided for Botulinum (3 area) treatment and 1 - 2 clients for filler to ensure that each of the key anatomical areas covered are observed. Morning = Botulinum (0900 - 1230) Afternoon = Botulinum and Option Dermal fillers (1330 - 1630) Conclusion Candidates given session and refreshments and discussion regarding Case Studies and further support. (1700 - 1800)
DEI Masterclass - Bringing The DEI Playbook To Life!
By Starling
This one day masterclass is designed to provide a practical application of the content that is covered within The DEI Playbook and is aimed at anyone tasked with launching and implementing diversity and inclusion within their organisation.

Still Technique Master Course
By CPD Today
Still Technique course, suitable for osteopaths and final year osteopathic students

We deliver Workplace PAT Testing Courses across most of the UK to assist businesses with Compliance. We also work with Bridges into Work and ReACT in association with Careers Wales and the Welsh Government to offer work based skills which some Candidates could be eligible for Government funding.

Permanent Makeup Laser Removal | N-Light-10 Laser Only
By ID Liner | Permanent Makeup Training & Supplies
HIGHLY SOUGHT-AFTER AND ALREADY AN EXTREMELY LUCRATIVE INDUSTRY, LASER TATTOO REMOVAL IS SET TO BE THE BIGGEST BEAUTY TREND OF 2023!

Permanent Makeup Laser Removal - Small Group Training - N-Light-10 Laser
By ID Liner | Permanent Makeup Training & Supplies
HIGHLY SOUGHT-AFTER AND ALREADY AN EXTREMELY LUCRATIVE INDUSTRY, LASER TATTOO REMOVAL IS SET TO BE THE BIGGEST BEAUTY TREND OF 2023!

Stand Up for Yourself (Communication skills and assertiveness)
5.0(6)By The Sunflower Effect Confidence Courses
Are challenging people getting in the way of your happiness, well-being and success? You may find them at work, in social situations, you may live them or they may be members of your family? Even worse when you see them getting away with things; getting their way even though there is no sense to it; getting advantages over everyone else; and in many cases being rewarded for their abusive behaviour with promotion and other advantages! You probably would never want to behave the way they do. You don’t have it in you or you just couldn’t live with yourself if you behaved like that. Maybe your attempts to confront them have failed and you’ve ended up with egg on your face, with them having more opportunity to have a “dig” at you. As a result, you may have come to the conclusion that it’s the nasty people that get ahead, and someone like you just has to put up with this behaviour from others.

This is a post-graduate course composed of a TCM and Acupuncture Theory Foundation Module (9 lecture days) and a Special Orthopaedic Acupuncture Module (8 lecture days) with additional 4 clinical practice days and a re- validation day. The TCM and Acupuncture Theory foundation Module leads to a Foundation Diploma in TCM and Acupuncture Level-1. The Orthopaedic Acupuncture module is designed in such a way that it can either be completed as a whole, or each of the 8 lectures can be taken as a CPD unit for therapist with special interests in the specific areas of the body. The course is specifically designed as an advanced, intensive course for acupuncturists, physiotherapists, osteopaths, chiropractors and other physical therapists who wish to broaden their knowledge and further their skills. The emphasis of the course falls on the practical application of acupuncture as a treatment for a variety of MSK conditions.

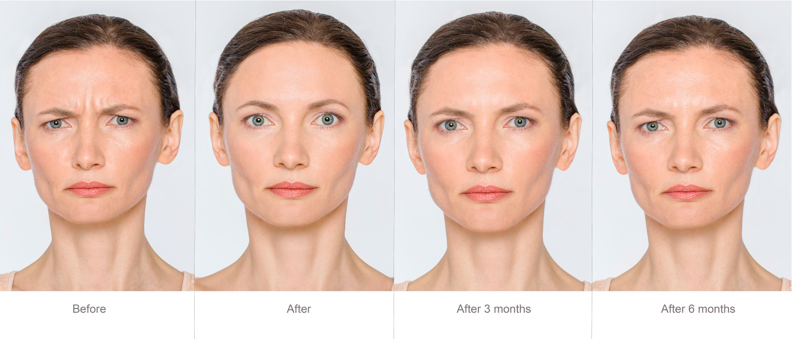
DERMAL FILLER COURSE
By Harley Elite Academy (HeLa)
Foundation • Advanced • Masterclass 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! Additional information ATTENDANCE ONLINE (theory), IN-CLINIC (Practice) COURSE LEVEL BEGINNER | Foundation Course, INTERMEDIATE | Advanced Course, EXPERT | Masterclass Course, ALL LEVELS 10% OFF
Search By Location
- CPD Courses in London
- CPD Courses in Birmingham
- CPD Courses in Glasgow
- CPD Courses in Liverpool
- CPD Courses in Bristol
- CPD Courses in Manchester
- CPD Courses in Sheffield
- CPD Courses in Leeds
- CPD Courses in Edinburgh
- CPD Courses in Leicester
- CPD Courses in Coventry
- CPD Courses in Bradford
- CPD Courses in Cardiff
- CPD Courses in Belfast
- CPD Courses in Nottingham
