- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Courses in Liverpool
We couldn't find any listings for your search. Explore our online options below.
Know someone teaching this? Help them become an Educator on Cademy.
Online Options
Show all 7PV03: Drug Safety and Pharmacovigilance
By Zenosis
Drug safety monitoring and risk management are vitally important for medicinal product developers, licence holders and clinical investigators. In addition to their duty to protect public health, increasingly tight regulation and potentially massive payments to litigants provide strong incentives for pharmaceutical and biotechnology companies to ensure that they maintain efficient systems for drug safety / pharmacovigilance and that all staff are aware of the basic requirements. This course will provide them with an overview of the most important aspects of this discipline, both before and after marketing of products, especially as they apply in Europe and the USA.

PV04: Signal Detection and Management in Pharmacovigilance
By Zenosis
This module provides a guide to signal detection and management for approved products. The subject is presented as a process comprising four stages: signal detection, signal validation, signal analysis and prioritisation, and risk assessment and minimisation.

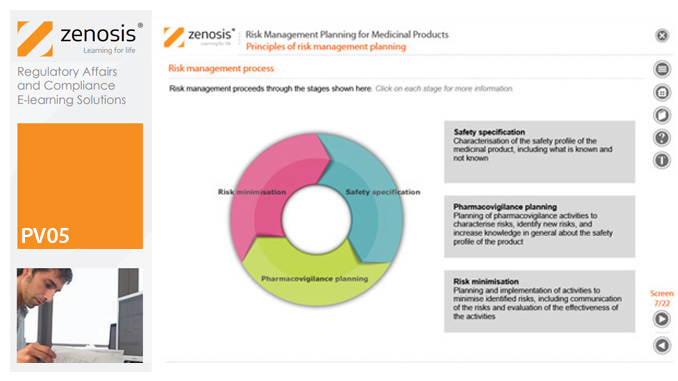
PV05: Risk Management Planning for Medicinal Products
By Zenosis
Proactive risk management is a major component of good pharmacovigilance practice. This module sets out the principles of risk management planning and outlines regulatory requirements for risk management plans in regions that are major markets for medicinal products.
This Diploma in Pharmacology at QLS Level 4 course is endorsed by The Quality Licence Scheme and accredited by CPDQS (with 120 CPD points) to make your skill development & career progression more accessible than ever! Are you familiar with Alexander Fleming's discovery of penicillin in 1928? That was the birth of modern pharmacology, which has since saved millions of lives worldwide. If you want to contribute to the ever-evolving field of medicine, then our Pharmacology Training course is the perfect opportunity for you. In this course, you will learn about the fundamental principles of pharmacology, drug development and regulation, neuropharmacology, chemotherapy, and more. Our comprehensive eight-module course covers a wide range of topics, including inflammation and immune pharmacology, and toxicology. You will have the opportunity to develop a deep understanding of pharmacology and learn how to apply your knowledge to real-world scenarios. This Pharmacology course will guide you through each module, ensuring that you have a strong grasp of each topic before moving on to the next one. By the end of this course, you will have a thorough understanding of pharmacology and be able to apply your knowledge to real-world scenarios. You will also develop the skills necessary to evaluate and analyse pharmacological data, understand drug development and regulation, and be able to work in teams to solve complex problems. This course will equip you with the knowledge and skills necessary to succeed in the field of pharmacology. After this Pharmacology Training course, you will be able to learn: Understand the fundamental principles of pharmacology Analyze drug development and regulation Evaluate the effects of neuropharmacology on the body Understand cardiovascular and endocrine pharmacology Analyze chemotherapy treatments for various diseases Understand inflammation and immune pharmacology Why Prefer This Pharmacology at QLS Level 5 Diplloma from HF Online? Pharmacology FREE certificate endorsed by the Quality Licence Scheme & another accredited by CPDQS Get a free student ID card! (£10 postal charge will be applicable for international delivery) with Pharmacology course Innovative and engaging content of Pharmacology Free assessments available with Pharmacology 24/7 tutor support available Get Lifetime Access Get Free CPD Certified PDF Certificate Take a step toward a brighter future! *** Course Curriculum of Pharmacology at QLS Level 4*** Module 01: Fundamental Principles of Pharmacology Module 02: Drug Development and Regulation Module 03: Neuropharmacology Module 04: Cardiovascular Pharmacology Module 05: Endocrine Pharmacology Module 06: Chemotherapy Module 07: Inflammation and Immune Pharmacologyc Module 08: Toxicology Assessment Process of Pharmacology at QLS Level 4 You have to complete the assignment questions given at the end of the course and score a minimum of 60% to pass each exam. Our expert trainers will assess your assignment and give you feedback after you submit the assignment. You will be entitled to claim a certificate endorsed by the Quality Licence Scheme after you have completed all of the Diploma in Pharmacology at QLS Level 4 exams. CPD 120 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Aspiring pharmacologists Medical students Pharmaceutical researchers Healthcare professionals Individuals interested in the field of medicine Science graduates Requirements No prior background or expertise is required to enrol on this course. Career path Pharmacologist Pharmaceutical Scientist Medical Science Liaison Clinical Research Associate Pharmacovigilance Officer Regulatory Affairs Manager Certificates CPDQS Accredited Certificate Digital certificate - Included Diploma in Pharmacology at QLS Level 4 Hard copy certificate - Included Show off Your New Skills with a Certificate of Completion After successfully completing the Diploma in Pharmacology at QLS Level 4, you can order an original hardcopy certificate of achievement endorsed by the Quality Licence Scheme andalso you can order CPDQSAccredited Certificate that is recognised all over the UK and also internationally. The certificates will be home-delivered, completely free of cost.

If you're looking to break into the world of clinical research, the Clinical Research Administration Fundamentals Course offers a straightforward and in-depth introduction to the field. This course will equip you with the essential knowledge and skills needed to navigate the administrative aspects of clinical research. From understanding the different stages of clinical trials to managing documentation and compliance requirements, you'll gain the expertise to support research teams efficiently. Whether you're new to the industry or seeking to build on existing knowledge, this course is designed to make complex topics clear and accessible. With the rapid growth of clinical research and its pivotal role in medical advancements, the need for skilled professionals is ever-present. This course will guide you through the fundamental principles, including ethics, safety protocols, and regulatory considerations. You'll learn how to manage research documentation, assist with trial set-ups, and support clinical study teams with precision. Plus, with the option to study remotely, you can complete the course at your own pace, making it ideal for those balancing work or other commitments. Get ready to enhance your understanding of this critical industry sector and open the door to career opportunities within clinical research. Key Features CPD Accredited FREE PDF + Hardcopy certificate Fully online, interactive course Self-paced learning and laptop, tablet and smartphone-friendly 24/7 Learning Assistance Discounts on bulk purchases Course Curriculum Module 01: Introduction to Clinical Research Administration Module 02: Clinical Trial Design and Planning Module 03: Ethics and Regulatory Compliance Module 04: Institutional Review Boards (IRBs) and Ethics Committees Module 05: Data Management and Recordkeeping Module 06: Safety Reporting and Adverse Events Module 07: Clinical Trial Monitoring and Auditing Module 08: Study Site Management and Quality Control Module 09: Data Analysis and Reporting Module 10: The Future of Clinical Research Learning Outcomes: Understand key principles in clinical trial planning and design. Demonstrate proficiency in navigating ethical and regulatory frameworks. Develop expertise in data management and rigorous recordkeeping. Implement safety reporting protocols and manage adverse events effectively. Conduct thorough clinical trial monitoring and auditing procedures. Acquire skills in study site management, quality control, and data analysis. Accreditation This course is CPD Quality Standards (CPD QS) accredited, providing you with up-to-date skills and knowledge and helping you to become more competent and effective in your chosen field. Certificate After completing this course, you will get a FREE Digital Certificate from Training Express. CPD 10 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Individuals aspiring to enter the field of clinical research. Healthcare professionals seeking to broaden their skill set. Graduates in life sciences or related disciplines. Research assistants and coordinators aiming for career advancement. Regulatory affairs professionals looking to specialise. Quality assurance personnel in healthcare and pharmaceutical sectors. Professionals transitioning to roles in clinical trial management. Anyone keen on staying ahead in the evolving landscape of clinical research. Career path Clinical Research Coordinator Regulatory Affairs Specialist Clinical Data Manager Quality Assurance Auditor Clinical Research Associate Pharmacovigilance Officer Certificates Digital certificate Digital certificate - Included Once you've successfully completed your course, you will immediately be sent a FREE digital certificate. Hard copy certificate Hard copy certificate - Included Also, you can have your FREE printed certificate delivered by post (shipping cost £3.99 in the UK). For all international addresses outside of the United Kingdom, the delivery fee for a hardcopy certificate will be only £10. Our certifications have no expiry dates, although we do recommend that you renew them every 12 months.

ICT02: Assuring Data Integrity in the Manufacture of Medicinal Products
By Zenosis
Pharmaceutical and biotechnology companies and researchers need to assure regulatory authorities of the reliability of the data that they generate or acquire during product development and manufacturing – that is, to demonstrate data integrity. Data integrity is assessed during regulatory inspections of manufacturing and research sites. Inadequacies of data integrity are frequently reported by inspectors and result in regulatory actions against the companies or individuals concerned. Practices that assure data integrity are required by law and/or expected by regulators in the fields of nonclinical and clinical research, manufacturing and distribution, and pharmacovigilance of medicinal products. This course explains the requirements and describes principles and practices that should be followed to assure regulators and contractual partners of data integrity in the manufacture of medicinal products.
AI Literacy for Pharma Professionals
By Academy Pharmaceutical Excellence
AI Literacy for Pharma Professionals – New Half-Day Masterclass 💊 Struggling to keep up with AI in the pharmaceutical world? From QPs to Regulatory Affairs, Pharmacovigilance to Manufacturing, this 3.5-hour course demystifies AI and shows how it applies to YOUR daily role. ✅ Understand core AI concepts ✅ Explore GxP-compliant applications ✅ Review MHRA, EMA & FDA guidance ✅ Learn how to safely evaluate and adopt AI tools ✅ Build inspection-ready, AI-literate teams 📚 Includes real-world case studies, interactive modules, and a roadmap for future readiness. Join us and gain the confidence to lead in a digitally transforming industry — no coding required!

Search By Location
- pharmacovigilance Courses in London
- pharmacovigilance Courses in Birmingham
- pharmacovigilance Courses in Glasgow
- pharmacovigilance Courses in Liverpool
- pharmacovigilance Courses in Bristol
- pharmacovigilance Courses in Manchester
- pharmacovigilance Courses in Sheffield
- pharmacovigilance Courses in Leeds
- pharmacovigilance Courses in Edinburgh
- pharmacovigilance Courses in Leicester
- pharmacovigilance Courses in Coventry
- pharmacovigilance Courses in Bradford
- pharmacovigilance Courses in Cardiff
- pharmacovigilance Courses in Belfast
- pharmacovigilance Courses in Nottingham