- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
1 Educators providing Courses in Liverpool
Courses matching "Documentation"
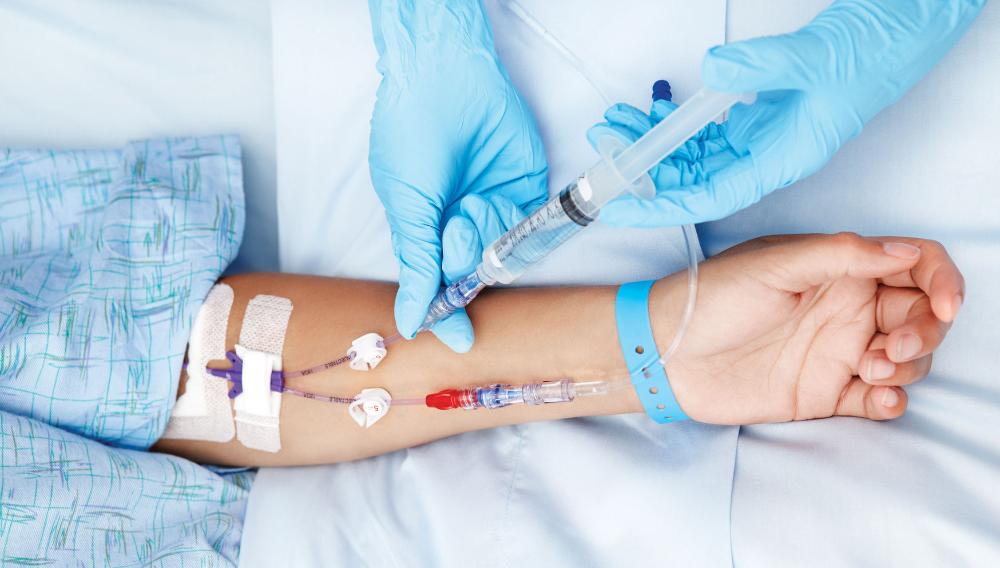
Show all 85Learn how to cannulate ... Nationally Recognised Qualification OCN Accredited - Level 3 (advanced) - Ireland Level 5 CPD Accredited - The CPD Certification Service Classroom or Virtual Classroom options Covers all steps for cannulating in arm or hand Practise on artificial arms and fake blood! Essential qualification for all IV therapies Phlebotomy training desirable but not essential Basic understanding of English language required OPEN TO ALL APPLICANTS
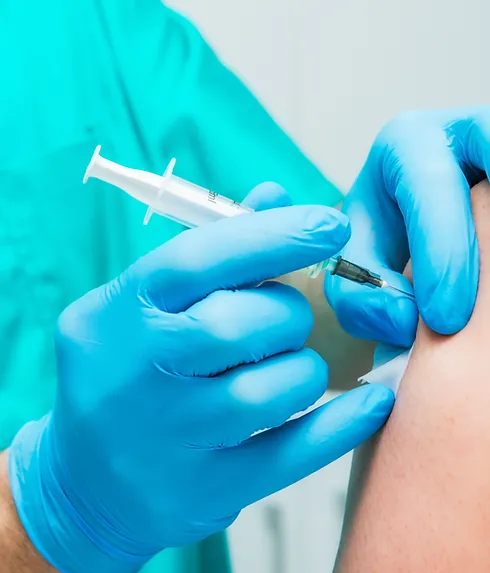
Learn how to administer vaccines or injections ... Nationally Recognised Qualification Includes IM, ID and Sub-Cut Injection methods OCN Accredited - Level 4 (Foundation Degree - FDSc) Covers all steps to safely perform a vaccination Use same techniques and skills for aesthetic therapies Includes B12, Vitamin C and other treatments Essential qualification for all injections Basic understanding of English language required OPEN TO ALL APPLICANTS
Documentation & Record Keeping for HCAs
By M&K Update Ltd
This session covers the legal aspects of documentation and record keeping in healthcare for Healthcare Assistants.

Documentation & Record Keeping for Nurses and AHPs
By M&K Update Ltd
This session covers the legal aspects of documentation and record keeping in healthcare.

Documentation & Record Keeping for Nurses and AHPs
By M&K Update Ltd
This session covers the legal aspects of documentation and record keeping in healthcare.

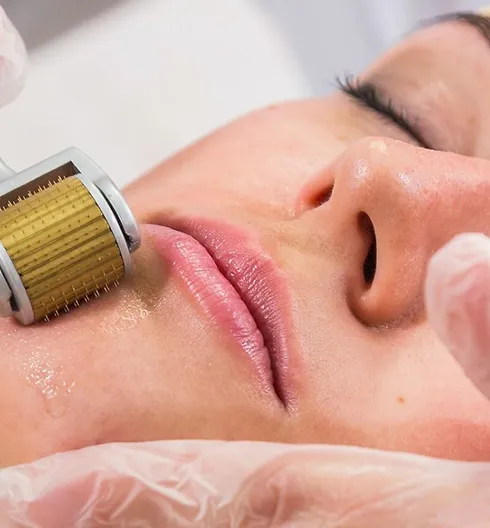
Platelet-rich Plasma (PRP) treatments Nationally Recognised Qualification No previous experience or qualifications needed Open College Network Accreditation Level 4 (as required for minimally invasive procedures) Covers standards set by HEE Employed (salon) or Self-Employed opportunities Basic understanding of English language required OPEN TO ALL APPLICANTS
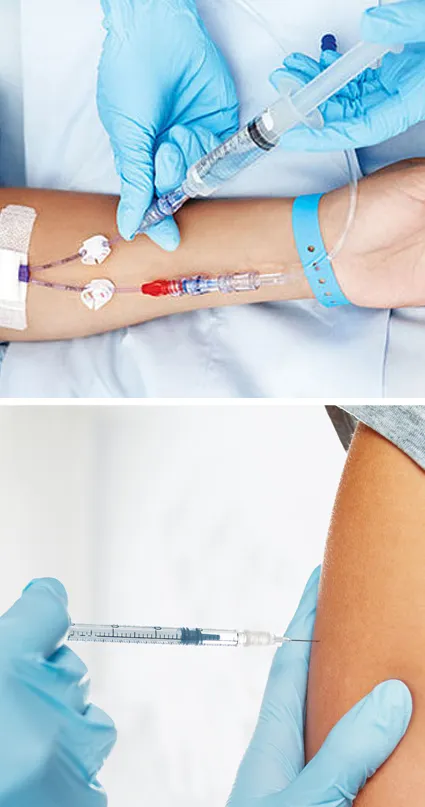
THIS COURSE PACKAGE INCLUDES: 1: PERIPHERAL I.V. CANNULATION - IV THERAPIES COURSE (GPT008) 2: VACCINATION / INJECTION COURSE (GPT601) Learn how to administer injectables and intravenous therapies ... FAST-TRACK YOUR AESTHETICS TRAINING WITH OUR COMPLETE TRAINING PACKAGE 20% Multi-Course Discount Cover all stages from Level 1 through to Level 4 (FDSc) Cover your theory training online Complete your advanced practical training in 1 day Practical training in Classroom or Virtual Classroom Comprehensive Practise@Home training kits for VC Awards 2 accredited qualifications Dual Accreditations for all courses Covers all steps required to safely perform injectables Covers all steps required to safely perform IV therapies Practise IV on artificial arm with fake blood Practise injection techniques on realistic injection pads Learn beginner to advanced skills and techniques Basic understanding of English language required OPEN TO ALL APPLICANTS
Writing and Managing Requirements Documents: In-House Training
By IIL Europe Ltd
Writing and Managing Requirements Documents: In-House Training This course is part of IIL's Business Analysis Certificate Program (BACP), a program designed to help prepare individuals pass the IIBA™ Certification exam to become a Certified Business Analysis Professional (CBAP™). Learn more at www.iil.com/bacp. Once a business analyst has completed the information gathering and analysis to produce the solution to a business problem, the results must be documented for all stakeholders to see and understand. This course will enhance the skill set needed for writing and managing the complex readership that business analysts interact with on a day-to-day basis. What you will Learn Upon completion, participants will be able to: Write an understood requirements document that is approvable and acceptable Validate a requirements document Manage the changes to requirements documents through the SDLC Foundation Concepts The role of the business analyst An introduction to the BABOK® Guide The business analyst and the product/project life cycle The requirements documentation process Planning for Effective Requirements Documentation Overview of requirements planning Planning for validation Planning for verification: well-formed criteria Planning for verification: understood and usable criteria Writing Effective Requirements Documents Overview of writing requirements documents Using a standard structure / template Applying formatting techniques Meeting the challenge of writing non-functional requirements Baselining Requirements Documents Overview of the requirements baseline process Validation Verification Approval Managing Requirements Change through the Product Life Cycle Overview of requirements change management Establishing a formal change management process Tracing requirements through design and development (build, test, and implementation) Following through to post-implementation (transition and early production)

An Understanding of Verification of Expected Death (Adult or Child as applicable) for Registered Nurses
By Guardian Angels Training
This verification of expected death training is suitable for nurses who are interested in taking on the extended role of verification of expected death within various healthcare settings.

Search By Location
- Documentation Courses in London
- Documentation Courses in Birmingham
- Documentation Courses in Glasgow
- Documentation Courses in Liverpool
- Documentation Courses in Bristol
- Documentation Courses in Manchester
- Documentation Courses in Sheffield
- Documentation Courses in Leeds
- Documentation Courses in Edinburgh
- Documentation Courses in Leicester
- Documentation Courses in Coventry
- Documentation Courses in Bradford
- Documentation Courses in Cardiff
- Documentation Courses in Belfast
- Documentation Courses in Nottingham

