- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
264 Courses in Coventry
Impact of Domestic Abuse in the Workplace (Gold Standard)
By Safe Space Consultancy
Creating safer, more aware workplaces through expert-led training covering domestic abuse, stalking, sexual harassment and safeguarding. Delivery Format: 2 full days OR modular segments Support Included: 9 hours of consultancy over 9 months Target Audience: HR Professionals, Line Managers, Senior Leaders, Wellbeing & DEI Leads Course Overview: This comprehensive training package equips organisations with the knowledge, tools, and confidence to recognise, respond to, and reduce the impact of domestic abuse within the workplace. Designed for HR professionals, line managers, and leadership teams, the course ensures your organisation is prepared to create a safe, supportive, and legally compliant work environment. With practical tools and follow-up support, this course empowers organisations to manage risk, comply with best practice, and make a meaningful difference in the lives of their employees. Course Modules: Introduction to Domestic Abuse Understand the definitions, forms, and prevalence of domestic abuse and its direct and indirect impact on the workplace. Implementing Domestic Abuse Policy and Procedures Guidance on developing and embedding clear, effective domestic abuse policies aligned with best practice and legal responsibilities. Spotting the Signs of Domestic Abuse Learn how to identify physical, behavioural, and performance-related indicators that an employee may be experiencing abuse. How to Support an Employee Build skills in approaching sensitive conversations, offering support without judgement, and connecting individuals to appropriate help. Understanding & Reducing Risk (to Employee and Business) Assess risks to the employee and the organisation, and implement safeguarding and protective strategies. Creating a Safe and Supportive Workplace Foster a culture of openness and safety through proactive measures, workplace adjustments, and employee engagement. Reporting, Confidentiality & Safety Planning Learn best practices for safe disclosures, confidentiality, and developing workplace safety plans. Digital Resources Access a curated collection of templates, checklists, signposting tools, and further reading to support long-term implementation. Ongoing Support: Participants receive 9 hours of consultancy or coaching over 9 months to assist with implementation, case discussions, or policy refinement. Please email julie@safespaceconsultancy.org for further information and to book a FREE 30 Consultation.

Facilitation Skills
By Elite Forums Events
Course Overview This practical, one-day course is designed to equip participants with the essential skills, tools and confidence to effectively facilitate discussions, meetings, and workshops. Whether you're guiding a team brainstorming session, leading a stakeholder workshop, or managing a complex meeting, strong facilitation skills can dramatically improve outcomes and engagement. Participants will learn how to plan and structure sessions, manage group dynamics, keep discussions on track, and handle challenging behaviours—all while creating a collaborative and inclusive environment. The course combines theory with hands-on activities, group discussions, and real-world scenarios to build practical, transferable skills. Who Should Attend This course is ideal for: Project managers Team leaders and supervisors Business analysts Community engagement officers Policy officers Trainers and consultants Anyone responsible for leading meetings or workshops No prior facilitation experience is necessary. Learning Outcomes By the end of the course, participants will be able to: Understand the role and mindset of an effective facilitator Plan and design structured facilitation sessions Use a range of facilitation tools and techniques to encourage participation Manage group dynamics, including difficult participants and off-topic conversations Apply active listening, summarising and questioning techniques Create safe, inclusive and engaging environments for diverse groups Maintain neutrality and guide discussions to achieve desired outcomes Course Content 1. Introduction to Facilitation What is facilitation? Key differences between facilitation, presentation and training The mindset of an effective facilitator 2. Planning for Success Clarifying session purpose and outcomes Structuring the session: openings, transitions, closings Selecting the right tools and approaches for your audience 3. Core Facilitation Techniques Questioning strategies (open, probing, clarifying) Active listening and reflection Encouraging balanced participation Visual facilitation basics (whiteboards, templates, sticky notes) 4. Managing Group Dynamics Reading the room and adapting your approach Handling dominant or disengaged participants Dealing with conflict or resistance constructively Techniques for decision-making and consensus-building 5. Practice and Feedback Facilitated role-plays and group exercises Constructive peer and trainer feedback Personal action planning Delivery Method This course is delivered in a highly interactive, face-to-face or virtual format. It includes a blend of short presentations, group work, facilitated discussions and hands-on activities to embed learning. Inclusions Comprehensive participant workbook and toolkit Facilitator guides and templates Certificate of completion Optional post-course coaching (available on request)

Facilitation Skills
By Elite Forums UK
Course Overview This practical, one-day course is designed to equip participants with the essential skills, tools and confidence to effectively facilitate discussions, meetings, and workshops. Whether you're guiding a team brainstorming session, leading a stakeholder workshop, or managing a complex meeting, strong facilitation skills can dramatically improve outcomes and engagement. Participants will learn how to plan and structure sessions, manage group dynamics, keep discussions on track, and handle challenging behaviours—all while creating a collaborative and inclusive environment. The course combines theory with hands-on activities, group discussions, and real-world scenarios to build practical, transferable skills. Who Should Attend This course is ideal for: Project managers Team leaders and supervisors Business analysts Community engagement officers Policy officers Trainers and consultants Anyone responsible for leading meetings or workshops No prior facilitation experience is necessary. Learning Outcomes By the end of the course, participants will be able to: Understand the role and mindset of an effective facilitator Plan and design structured facilitation sessions Use a range of facilitation tools and techniques to encourage participation Manage group dynamics, including difficult participants and off-topic conversations Apply active listening, summarising and questioning techniques Create safe, inclusive and engaging environments for diverse groups Maintain neutrality and guide discussions to achieve desired outcomes Course Content 1. Introduction to Facilitation What is facilitation? Key differences between facilitation, presentation and training The mindset of an effective facilitator 2. Planning for Success Clarifying session purpose and outcomes Structuring the session: openings, transitions, closings Selecting the right tools and approaches for your audience 3. Core Facilitation Techniques Questioning strategies (open, probing, clarifying) Active listening and reflection Encouraging balanced participation Visual facilitation basics (whiteboards, templates, sticky notes) 4. Managing Group Dynamics Reading the room and adapting your approach Handling dominant or disengaged participants Dealing with conflict or resistance constructively Techniques for decision-making and consensus-building 5. Practice and Feedback Facilitated role-plays and group exercises Constructive peer and trainer feedback Personal action planning Delivery Method This course is delivered in a highly interactive, face-to-face or virtual format. It includes a blend of short presentations, group work, facilitated discussions and hands-on activities to embed learning. Inclusions Comprehensive participant workbook and toolkit Facilitator guides and templates Certificate of completion Optional post-course coaching (available on request)

Maximizing Academic Success: How to Use a Free Assignment Sample in UK
By david hude
This article explores the advantages of using a Free Assignment Sample in UK to improve academic performance. It highlights how New Assignment Help provides valuable resources tailored to UK academic standards, assisting students in creating well-structured, high-quality assignments.

Online Options
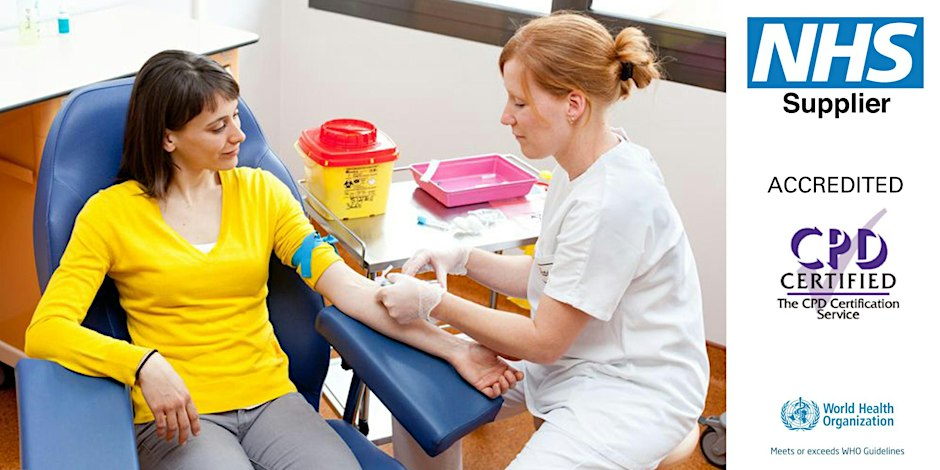
Show all 18930THIS COURSE PACKAGE INCLUDES: 1: INTRODUCTION TO PHLEBOTOMY COURSE (GPT003) - Level 3 (Ireland Level 5) 2: ADVANCED PHLEBOTOMY COURSE (GPT005) - Level 4 (Ireland Level 6) 3: GEOPACE COMPETENCY CERTIFICATE - CPD Certified (optional with Virtual Classroom) Learn how to take blood ... train as a Phlebotomist FAST-TRACK YOUR PHLEBOTOMY TRAINING WITH OUR COMPLETE TRAINING PACKAGE 20% off - Multi-Course Discount Cover all stages from beginner through to Level 4 Available as Classroom or Virtual Classroom Complete your beginner to advanced training in 2 days Awards 2 accredited qualifications - Introduction to Phlebotomy and Advanced Phlebotomy qualifications Both courses are dually accredited (OCN & CPD) Geopace Certificate of Competency included with classroom attendance or available as an option when booking virtual classroom Covers all steps up to live blood draw Learn advanced skills and techniques Virtual Classroom options include comprehensive Practise@Home Training Kits (yours to keep) Basic understanding of English language required OPEN TO ALL APPLICANTS
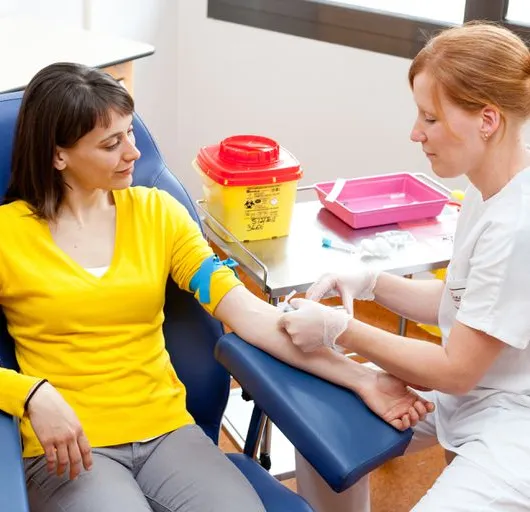
The UK's first and only Level 4 qualification in Phlebotomy (equivalent to Ireland Level 6) FDSc (Foundation Degree Level) qualification Nationally Recognised certificate Dually accredited: Open College Network and CPD Covers both aspirated and evacuated systems Covers specialised blood collection systems & methods Classroom or Virtual Classroom learning options Comprehensive Training Kit is provided when booking our Virtual Classroom option (yours to keep) Complete your training from beginner to advanced level This course either follows on from our Introduction to Phlebotomy Course or can be combined with our introductory course as part of a course package (see below) Available to candidates who have completed (or are currently enrolled to complete) our Introduction to Phlebotomy Course or have previous phlebotomy practical experience.
Embark on a mesmerising journey with 'Introduction to Mythology: Gods and Heroes'. Traverse through time, exploring the hallowed halls of Ancient Greece, the echoing chambers of Norse legends, and the sun-drenched tales of Egypt. Let each module introduce you to divine pantheons, fearless heroes, and the tapestries they've woven into our world's cultural fabric. By understanding the myths, stories, and legends of various cultures, you'll unlock a deeper appreciation for the human psyche and the narratives that shape us. Learning Outcomes Discover the foundational myths from diverse global cultures. Analyse and differentiate between mythological tales from different eras and regions. Recognise the influence of mythology in contemporary societies. Explore the art of mythological interpretation and its significance. Gain insights into the shared themes across various mythologies. Why choose this Introduction to Mythology: Gods and Heroes course? Unlimited access to the course for a lifetime. Opportunity to earn a certificate accredited by the CPD Quality Standards after completing this course. Structured lesson planning in line with industry standards. Immerse yourself in innovative and captivating course materials and activities. Assessments are designed to evaluate advanced cognitive abilities and skill proficiency. Flexibility to complete the Introduction to Mythology: Gods and Heroes Course at your own pace, on your own schedule. Receive full tutor support throughout the week, from Monday to Friday, to enhance your learning experience. Who is this Introduction to Mythology: Gods and Heroes course for? Storytellers and writers seeking to enrich their narrative depth. History buffs keen on exploring cultural legacies. Students of literature wanting to broaden their understanding. Curious minds eager to delve into age-old tales and legends. Educators aiming to introduce diverse mythologies in their curriculum. Career path Mythology Researcher: £25,000 - £40,000 Historical Content Creator: £28,000 - £45,000 Cultural Studies Lecturer: £35,000 - £50,000 Mythological Consultant for Media: £30,000 - £48,000 Folklore and Mythology Writer: £24,000 - £39,000 Museum Curator (Mythological Exhibits): £29,000 - £46,000 Prerequisites This Introduction to Mythology: Gods and Heroes does not require you to have any prior qualifications or experience. You can just enrol and start learning.This Introduction to Mythology: Gods and Heroes was made by professionals and it is compatible with all PC's, Mac's, tablets and smartphones. You will be able to access the course from anywhere at any time as long as you have a good enough internet connection. Certification After studying the course materials, there will be a written assignment test which you can take at the end of the course. After successfully passing the test you will be able to claim the pdf certificate for £4.99 Original Hard Copy certificates need to be ordered at an additional cost of £8. Course Curriculum Module 01: Introduction to Mythology Introduction to Mythology 00:12:00 Module 02: Mythology of Ancient Greece Mythology of Ancient Greece 00:11:00 Module 03: Mythology of Ancient Rome Mythology of Ancient Rome 00:12:00 Module 04: Norse Mythology Norse Mythology 00:10:00 Module 05: Egyptian Mythology Egyptian Mythology 00:11:00 Module 06: Mythology of the Americas Mythology of the Americas 00:13:00 Module 07: Mythology in Other Cultures Mythology in Other Cultures 00:11:00 Module 08: Mythological Interpretation Mythological Interpretation 00:10:00

Embark on an epic exploration of gods and heroes across cultures with our 'Introduction to Mythology' course. Dive into the enchanting realms of Ancient Greece, Rome, Norse, Egyptian, and Americas mythologies. Uncover universal themes, analyze cultural nuances, and develop a profound understanding of mythological interpretation. Join us on a journey through time and imagination, discovering the stories that have shaped civilizations and continue to resonate in today's global tapestry of human experience.

Take your phlebotomy qualifications to the next level ... Nationally Recognised Qualification OCN Accredited - Level 3 (advanced) CPD Accredited Covers specialised and advanced phlebotomy techniques and practices Comprehensively covers Peripheral IV Cannulation Advanced qualification - additional credits Download a digital certificate on completion Basic understanding of English language required LOOKING TO ADD PRACTICAL TRAINING? ALSO AVAILABLE AS SEPARATE CLASSROOM OR VIRTUAL CLASSROOM COURSES: 1: Advanced Phlebotomy Course - Level 4 2: Peripheral IV Cannulation Course - Level 3 COMPLETION OF INTRODUCTION TO PHLEBOTOMY COURSE RECOMMENDED BUT NOT ESSENTIAL
Specialised techniques and skills associated with neonatal and paediatric blood draws Nationally Recognised Qualification OCN Accredited - Level 3 (advanced) CPD Accredited - The CPD Certification Service Follow-on from Introduction to Phlebotomy Course Complements our Advanced Phlebotomy Course (Level 4 - FDSc level) Expand your horizons and add new skills Covers neonates, infants and child draws Legal framework and consenting Download a certificate on completion of your online course FOLLOWS ON FROM INTRODUCTION TO PHLEBOTOMY COURSE BUT ALSO OPEN TO ALL APPLICANTS
Understanding specific words and terms used in the healthcare sector is an absolute must if you are looking to progress in this profession. Once you have a basic understanding of how medical words are constructed they become easy to understand and use are internationally used by nurses, doctors, allied healthcare professionals, dentists and many other medical specialities.

Search By Location
- Introduction to Mythology Courses in London
- Introduction to Mythology Courses in Birmingham
- Introduction to Mythology Courses in Glasgow
- Introduction to Mythology Courses in Liverpool
- Introduction to Mythology Courses in Bristol
- Introduction to Mythology Courses in Manchester
- Introduction to Mythology Courses in Sheffield
- Introduction to Mythology Courses in Leeds
- Introduction to Mythology Courses in Edinburgh
- Introduction to Mythology Courses in Leicester
- Introduction to Mythology Courses in Coventry
- Introduction to Mythology Courses in Bradford
- Introduction to Mythology Courses in Cardiff
- Introduction to Mythology Courses in Belfast
- Introduction to Mythology Courses in Nottingham