- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
23245 Improv courses
ISO 27701 Lead Implementer
By Training Centre
Delivered in either our Live Online (4 days) or in our Classroom (5 days), the ISO/IEC 27701 Lead Implementer training course enables you to develop the necessary expertise to assist an organization to establish, implement, maintain and continually improve a Privacy Information Management System (PIMS) based on ISO/IEC 27701 by enhancing an existing ISMS based on ISO/IEC 27001 and the guidance of ISO/IEC 27002. About This Course This training course is designed to prepare its participants implement a Privacy Information Management System (PIMS) in compliance with the requirements and guidance of the ISO/IEC 27701. Moreover, you will gain a comprehensive understanding of the best practices of privacy information management and learn how to manage and process data while complying with various data privacy regimes. After mastering the implementation and management of a Privacy Information Management System (PIMS), you can sit for the exam and gain the "Certified ISO/IEC 27701 Lead Implementer' credential. The internationally recognized Certificate proves that you have the practical knowledge and professional capabilities to implement the ISO/IEC 27701 requirements in an organization. Learning objectives Master the concepts, approaches, methods and techniques used for the implementation and effective management of a PIMS. Learn about the correlation between ISO/IEC 27701, ISO/IEC 27001, ISO/IEC 27002 and other standards and regulatory frameworks. Understand the operation of a PIMS based on ISO/IEC 27701 and its principal processes. Learn how to interpret the requirements of ISO/IEC 27701 in the specific context of an organization. Develop the expertise to support an organization in effectively planning, implementing, managing, monitoring and maintaining a PIMS. Education approach This training course is based on both theory and best practices used in the implementation of PIMS. Lecture sessions are illustrated with examples based on case studies. Practical exercises are based on a case study which includes role playing and discussions. Practice tests are similar to the Certification Exam Prerequisites A fundamental understanding of information security and comprehensive knowledge of the ISMS implementation principles What's Included? Refreshments & Lunch (Classroom courses only) Course Slide Deck Official Study Guides CPD Certificate The Exam fees Who Should Attend? Managers and consultants involved in privacy and data management Expert advisors seeking to master the implementation of a Privacy Information Management System Individuals responsible and accountable for Personally Identifiable Information (PII) within organizations Individuals responsible for maintaining conformance with data privacy regimes requirements PIMS team members Accreditation Our Guarantee We are an approved IECB Training Partner. You can learn wherever and whenever you want with our robust classroom and interactive online training courses. Our courses are taught by qualified practitioners with commercial experience. We strive to give our delegates the hands-on experience. Our courses are all-inclusive with no hidden extras. The one-off cost covers the training, all course materials, and exam voucher. Our aim: To achieve a 100% first time pass rate on all our instructor-led courses. Our Promise: Pass first time or 'train' again for FREE. *FREE training and exam retake offered Assessment The exam consists of a 12 question essay type format, to be completed within 150 minutes and achieve a pass mark of 70%. Exam results are provided within 24 hours. Provided by This course is Accredited by NACS and Administered by the IECB.

ISO 37301 Lead Implementer
By Training Centre
A CMS provides organizations a structured approach to meet all compliance obligations, i.e., requirements that they mandatorily have to comply with such as laws, regulations, court rulings, permits, licenses, as well as those that they voluntarily choose to comply with such as internal policies and procedures, codes of conduct, standards, and agreements with communities or NGOs. About This Course The benefits of implementing a compliance management system (CMS) based on ISO 37301 are manifold: helping the organization avoid or mitigate the costs, risks, and damage of noncompliance, ensuring the long-term sustainability of the organization, promoting trust and confidence, encouraging good governance practices, due diligence, and ethically sound business dealings, etc. The ISO 37301 Lead Implementer training course provides the knowledge needed to establish, implement, manage, maintain, and continually improve a CMS. It aims to provide an in-depth understanding of ISO 37301 requirements, as well as the best practices and approaches used for the implementation and subsequent maintenance of the compliance management system. The training course enables you to help organizations establish processes needed to adhere to all compliance obligations and establish controls that proactively prevent noncompliance and contribute to the creation of a culture of integrity, transparency, and openness. The training course is followed by the certification exam. If you pass, you gain the 'Certified ISO 37301 Lead Implementer' credential. This credential validates your professional capabilities and competences to implement a CMS in an organization based on the requirements of ISO 37301. This training course will help you: Gain a comprehensive understanding of the concepts, approaches, methods, and techniques used for the implementation and effective management of a CMS Acknowledge the correlation between ISO 37301 and other standards and regulatory frameworks Gain the ability to interpret the requirements of ISO 37301 in the specific context of an organization Develop the necessary knowledge and expertise to support an organization in effectively planning, implementing, managing, monitoring, and maintaining a CMS Acquire the expertise to advise an organization in implementing CMS best practices Assessment Delegates sit a combined exam, consisting of in-course quizzes and exercises, as well as a final 12 question, essay type exam on Day 4 of the course. The overall passing score is 70%, to be achieved within the 150 minute time allowance. Exam results are provided within 24 hours, with both a Certificate and a digital badge provided as proof of success. Prerequisites The main requirements for participating in this training course are a basic knowledge of ISO management system standards, as well as a general understanding of ISO 37301 (or ISO 19600 guidelines) and the MS implementation principles. What's Included? Certification fees are included in the exam price. Training material of over 450 pages of information and practical examples will be provided. An attestation of course completion worth 31 CPD (Continuing Professional Development) credits will be issued to participants who have attended the training course. In case of exam failure, candidates can retake the exam once for free within 12 months following the initial exam date. Who Should Attend? Managers, consultants, and compliance officers wishing to develop a thorough understanding of ISO 37301 requirements for a compliance management system Managers and consultants seeking a comprehensive CMS implementation framework Compliance officers responsible for practicing due diligence with regard to compliance risks Individuals wishing to contribute in maintaining organizational integrity by supporting ethical behaviour Managers and members of governance, risk management, and compliance teams Individuals aspiring to become compliance officers or compliance management consultant Accreditation Provided by This course is Accredited by NACS and Administered by the IECB

ISO 27035 Lead Incident Manager
By Training Centre
During this training course, you will gain a comprehensive knowledge of a process model for designing and developing an organizational incident management plan. The compatibility of this training course with ISO/IEC 27035 also supports the ISO/IEC 27001 by providing guidance for Information Security Incident Management. After mastering all the necessary concepts of Information Security Incident Management, you can sit for the exam and gain "Certified ISO 27035 Lead Incident Manager" Certification. By holding this certification, you will be able to demonstrate that you have the practical knowledge and professional capabilities to support and lead a team in managing Information Security Incidents. About This Course Learning objectives Master the concepts, approaches, methods, tools and techniques that enable an effective Information Security Incident Management according to ISO/IEC 27035 Acknowledge the correlation between ISO/IEC 27035 and other standards and regulatory frameworks Acquire the expertise to support an organization to effectively implement, manage and maintain an Information Security Incident Response plan Acquire the competence to effectively advise organizations on the best practices of Information Security Incident Management Understand the importance of establishing well-structured procedures and policies for Incident Management processes Develop the expertise to manage an effective Incident Response Team Course Agenda Day 1: Introduction to Information Security Incident Management concepts as recommended by ISO/IEC 27035 Day 2: Designing and preparing an Information Security Incident Management plan Day 3: Enacting the Incident Management process and handling Information Security incidents Day 4: Monitoring and continual improvement of the Information Security Incident Management plan and the Exam. Additional Information Certification fees are included in the exam price. An attendance record worth 31 CPD (Continuing Professional Development) credits will be issued to the participants who have attended the training course. In case candidates fail the exam, they can retake it within 12 months of the initial attempt for free. Accreditation Assessment Delegates sit a combined exam, consisting of in-course quizzes and exercises, as well as a final 12 question, essay type exam on Day 4 of the course. The overall passing score is 70%, to be achieved within the 150 minute time allowance. Exam results are provided within 24 hours, with both a Certificate and a digital badge provided as proof of success. Our Guarantee We are an Accredited Training Provider of the IECB. You can learn wherever and whenever you want with our robust classroom and interactive online training courses. Our courses are taught by qualified practitioners with a minimum of 25 years commercial experience. We strive to give our delegates the hands-on experience. Our courses are all-inclusive with no hidden extras. The one-off cost covers the training, all course materials, and exam voucher. Our aim: To achieve a 100% first time pass rate on all our instructor-led courses. Our Promise: Pass first time or 'train' again for FREE. *FREE training offered for retakes - come back within a year and only pay for the exam. Prerequisites A fundamental understanding of ISO/IEC 27035 and comprehensive knowledge of Information Security. What's Included? Delegates will be provided with; Course Slide deck Questions and Answers Bank Participant Guide Who Should Attend? Information Security Incident managers IT Managers IT Auditors Managers seeking to establish an Incident Response Team (IRT) Managers seeking to learn more about operating effective IRTs Information Security risk managers IT system administration professionals IT network administration professionals Members of Incident Response Teams Individuals responsible for Information Security within an organization Provided by This course is Accredited by NACS and Administered by the IECB

ISO 27001 (2022) Lead Auditor
By Training Centre
Delivered in either Live Online (4 days) or in our Classroom (5 days), the ISO/IEC 27001 Lead Auditor training enables you to develop the necessary expertise to support an organization in establishing, implementing, managing and maintaining an Information Security Management System (ISMS) based on ISO 27001. During this training course, you will acquire the knowledge and skills to plan and carry out internal and external audits in compliance with ISO 19011 and ISO/IEC 17021-1 certification process. About This Course Based on practical exercises, you will be able to master audit techniques and become competent to manage an audit program, audit team, communication with customers, and conflict resolution. After acquiring the necessary expertise to perform this audit, you can sit for the exam and gain the "ISO/IEC 27001 Lead Auditor' credential. By holding this Lead Auditor Certificate, you will demonstrate that you have the capabilities and competencies to` audit organizations based on best practices. The training course is based on both theory and best practices used in ISMS audits Lecture sessions are illustrated with examples based on case studies Practical exercises are based on a case study which includes role playing and discussions Practice tests are similar to the Certification Exam The course is delivered both as a Live Online or Classroom environment, as follows; Day 1: Introduction to Information Security Management Systems (ISMS) and ISO/IEC 27001 Day 2: Audit principles, preparation and launching of an audit Day 3: On-site audit activities Day 4: Closing the audit and Examination Learning Objectives Understand the operations of an Information Security Management System based on ISO/IEC 27001 Acknowledge the correlation between ISO/IEC 27001, ISO/IEC 27002 and other standards and regulatory frameworks Understand an auditor's role to: plan, lead and follow-up on a management system audit in accordance with ISO 19011 Learn how to lead an audit and audit team Learn how to interpret the requirements of ISO/IEC 27001 in the context of an ISMS audit Acquire the competencies of an auditor to: plan an audit, lead an audit, draft reports, and follow-up on an audit in compliance with ISO 19011 The exam covers the following competency domains: Domain 1: Fundamental principles and concepts of an Information Security Management System (ISMS) Domain 2: Information Security Management System controls and best practices based on ISO/IEC 27002 Domain 3: Planning an ISMS implementation based on ISO/IEC 27001 Domain 4: Implementing an ISMS based on ISO/IEC 27001 Domain 5: Performance evaluation, monitoring and measurement of an ISMS based on ISO/IEC 27001 Domain 6: Continual improvement of an ISMS based on ISO/IEC 27001 Domain 7: Preparing for an ISMS certification audit Prerequisites A foundational understanding of ISO/IEC 27001 and knowledge of audit principles. What's Included? Refreshments & Lunch (Classroom only) Course Slide Deck Official Study Materials CPD Certificate The Exam Who Should Attend? Auditors seeking to perform and lead Information Security Management System (ISMS) certification audits Managers or consultants seeking to master an Information Security Management System audit process Individuals responsible for maintaining conformance with Information Security Management System requirements Technical experts seeking to prepare for an Information Security Management System audit Expert advisors in Information Security Management Our Guarantee We are an approved IECB Training Partner for all of our courses. You can learn wherever and whenever you want with our robust classroom and interactive online training courses. Our courses are taught by qualified practitioners with commercial experience. We strive to give our delegates the hands-on experience. Our courses are all-inclusive with no hidden extras. The one-off cost covers the training, all course materials, and exam voucher. Our aim: To achieve a 100% first time pass rate on all our instructor-led courses. Our Promise: Pass first time or 'train' again for FREE. *FREE training and exam retake offered Accreditation Assessment Delegates sit a combined exam, consisting of in-course quizzes and exercises, as well as a final 12 question, essay type exam on Day 4 of the course. The overall passing score is 70%, to be achieved within the 240 minute time allowance. Exam results are provided within 24 hours, with both a Certificate and a digital badge provided as proof of success. Provided by This course is Accredited by NACS and Administered by the IECB

The Visual Factory - Fundamentals of Visual Management in Manufacturing
By Centre for Competitiveness
Understand the principles of the “Visual Factory”, basic “Visual Management Systems” and how to apply it to your working environment This workshop introduces the tools and practices of Visual Management as one of the basic components of a Lean Management System. It shows how it can be used effectively to support standardization and problem solving across all functions, allowing the detection of product and process abnormalities in your value stream in real time. It also serves as a communication tool by visually defining for people what "good" is, what the "standard" is, and whether it is being maintained. It allows a proactive approach to data gathering, leading to rapid data analysis, and aids decision making that results in corrective actions being taken in a cost effective way. To achieve this, the workshop looks at how to identify critical product and process information, how to record and display it, who needs to see it, and how does it lead to action that resolves a problem or a non-conformance when it occurs. The workshop also details the principles used in designing and implementation a "Visual Management System" in order to migrate your organization from one of gathering and analysing data, and reacting to abnormalities, to one of seeing waste, managing exceptions, and improving processes in real time.

If you prefer the convenience of learning from home, or live outside the UK, join our online hypnotherapy training. We welcome students from all over the world* and you can study at either Foundation or Practitioner levels. If you want to train during the week, our midweek class offers a blended option, including both online and in-person students. This involves attending for one full day, then thirty-eight half days (one per week in term time only). It starts in September, so the Foundation level ends in January and the Practitioner level in July. Availability of blended places is limited, so please complete the enrolment form in good time.

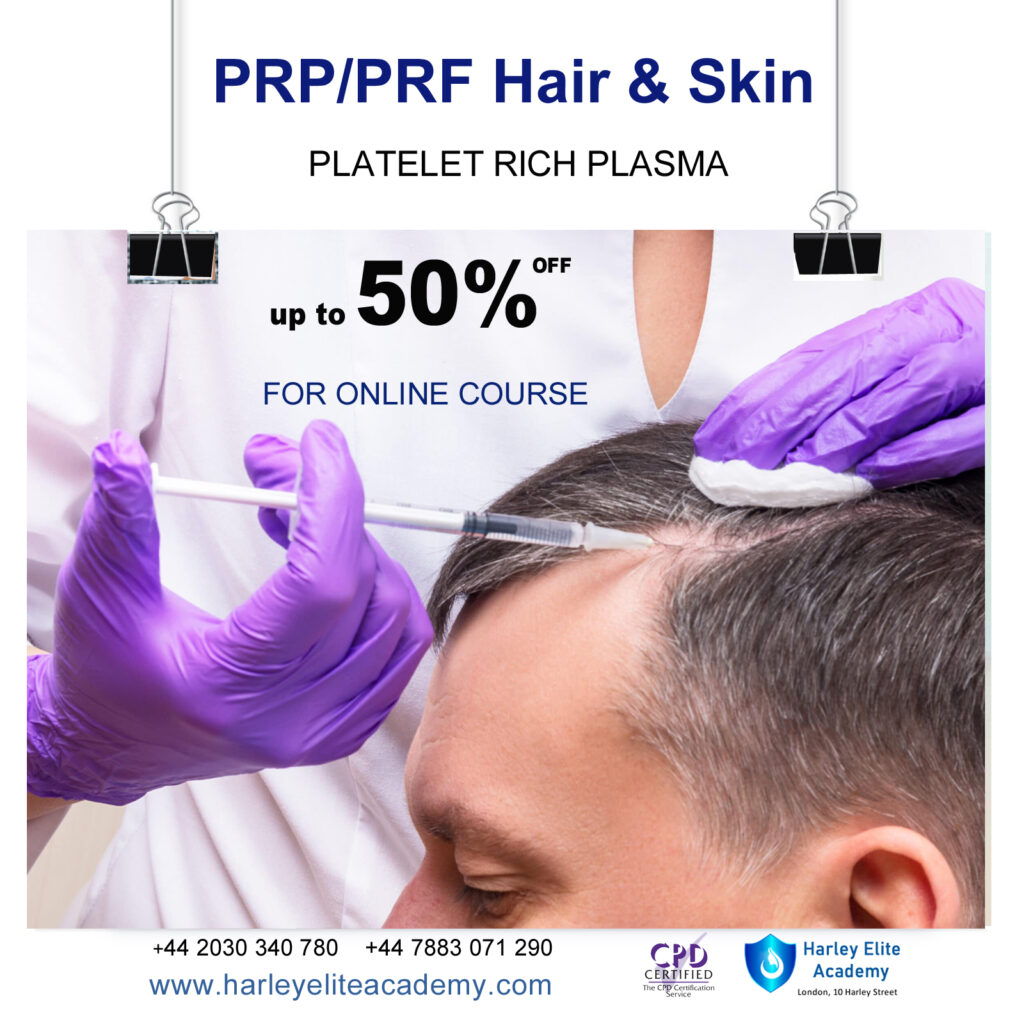
PRP / PRF SKIN & HAIR COURSE
By Harley Elite Academy (HeLa)
MASTERCLASS 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! CLINICAL PRP • Sports medicine • Traumatology • Ophthalmic • Burn trauma • Wound healing –diabetic foot • Skin grafting • Dentistry-sinus lift • Tooth implants. PRP theory & equipment: Training Online Theory will enable you to understand: Anatomy Vascular Supply, Contraindications Patient consultation Complications Management Post treatment advice Dealing with equipment A certification of training will be provided upon completion of the course. Aesthetic PRP • Skin rejuvenation • Hair restoration • Fat grafting in combination PRP • Post laser •Acne & Rosacea •Acne scar •TissueVolumisation alternative of HA fillers •Aesthetic gynecology /urology. Plathelet Rich Plasma We will cover pertinent information including mechanism of action, safety and efficacy issues, management and treatment of complications, dilution guidelines, and more. Hands on practical session – skin rejuvenation and hair loss Extraction, Preparation and Dosage Management Injection techniques – face, neck and head (hair loss); also the use of cannula Upon successful completion of the course, you will receive a certificate and title of PRP Certified Practitioner. MASTER CLASS PRP & PRF During the course we are providing . Taking blood and how to use a Centrifuge . PRP injecting techniques in face neck and décolletage hands. PRP Microneedling using a DERMAPEN. Combination treatment PRP with Mesotherapy. MECHANISM OF ACTION Platelets + Leucocytes form 3D mesh release of GF Chemo attraction and migration of macrophages and stem cells Stem cells proliferates by mitosis Stem cells undergo differentiation process BENEFIT FROM PRP TREATMENT & THERAPYExperience the advantages of PRP treatment and therapy, utilizing autologous blood with natural growth factors for disease-free and hypoallergenic benefits. Boost wound healing by regulating mitosis, proliferation, and differentiation, enhancing tissue with collagen, elastin, and hyaluronic acid. Benefit from improved tissue oxygenation, nutrition flow, and support for procedures like hair transplants, fat transfers, and skin grafts.PRP works effectively in skin rejuvenation, facial resurfacing, microneedling, and combines well with HA, PDO threads, skin boosters, peeling, or CO2 lasers. It also proves beneficial for hair restoration, showing positive results in various protocols for Androgenic alopecia and age-related hair loss.PRP where works .Skin rejuvenation-facial resurfacing.application-injection alone. Microneedling Combination with HA,Combination with PDO threads,Skin boosters , peeling or CO2 lasers Hair restoration, Multiple protocols with positive results Evidence for improvement of: Androgenic alopecia-male and females, “spot hair lost” Improvement of age related hair loss. You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (Theory), IN CLINIC (Practice) COURSE LEVEL EXPERT | Masterclass Course
M.D.D SCHEMA THERAPY PACKAGE (SELF IMPROVEMENT)
4.9(27)By Miss Date Doctor Dating Coach London, Couples Therapy
Schema Therapy Package: Break Free from Old Patterns, Cultivate Emotional Well-being Discover the transformative potential of Schema Therapy with Miss Date Doctor’s specialized package. Schema therapy integrates cognitive-behavioural techniques with elements of psychodynamic therapy, aiming to identify and modify long-standing patterns or schemas that contribute to emotional and interpersonal difficulties. Under the guidance of our skilled therapists, you’ll explore deep-rooted patterns and learn to challenge and change unhelpful behaviors and beliefs. As you break free from old patterns, you’ll cultivate emotional well-being and create healthier relationships. Uncover your true self and experience profound personal growth with the Schema Therapy package. 3 X1 hour session https://relationshipsmdd.com/product/schema-therapy-package/

M.D.D THERAPY FOR REPRESSED EMOTIONS PACKAGE (SELF IMPROVEMENT)
4.9(27)By Miss Date Doctor Dating Coach London, Couples Therapy
Introducing Therapy for Repressed Emotions Package: Unlocking Emotional Freedom and Healing Are you struggling with repressed emotions, finding it difficult to express and process your feelings from the past? Miss Date Doctor’s Therapy for Repressed Emotions Package offers you compassionate support and guidance to unlock emotional freedom, heal past wounds, and embrace a healthier emotional state. Repressed emotions are feelings that have been pushed aside or buried due to various reasons, leading to unresolved emotional baggage. Our experienced therapists are dedicated to helping you navigate through these suppressed emotions, fostering emotional release, and promoting healing. Here’s how the Therapy for Repressed Emotions Package can support you: Understanding Repressed Emotions: Our therapists will help you understand the nature of repressed emotions and how they impact your current emotional well-being. Creating a Safe Space: We offer a safe and non-judgmental environment where you can freely explore and express your suppressed emotions. Emotional Release: Therapy will focus on facilitating emotional release, allowing you to process and express feelings that have been held back. Healing Past Wounds: We’ll work on addressing and healing the unresolved emotional wounds from the past. Coping Strategies: Our therapists will equip you with healthy coping strategies to manage overwhelming emotions as they arise. Self-Compassion and Acceptance: Therapy encourages self-compassion and self-acceptance as you navigate through your emotional journey. Building Emotional Resilience: We’ll focus on developing emotional resilience to face and process emotions in a healthy way. Mindfulness Practices: Therapy for repressed emotions may incorporate mindfulness techniques to cultivate present-moment awareness. The Therapy for Repressed Emotions Package at Miss Date Doctor is designed to empower you to release the emotional burden of the past and find emotional freedom. Our compassionate therapists provide gentle guidance and support to help you navigate through your emotional journey. Invest in your emotional well-being and take the first step towards unlocking emotional freedom with the Therapy for Repressed Emotions Package. Embrace the opportunity to heal past wounds, express your feelings, and foster a healthier emotional state. Let our experienced therapists guide you towards emotional release, healing, and a brighter emotional future. 8 SESSIONS X 1 HOUR https://relationshipsmdd.com/product/therapy-for-repressed-emotions-package/

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Search By Location
- Improv Courses in London
- Improv Courses in Birmingham
- Improv Courses in Glasgow
- Improv Courses in Liverpool
- Improv Courses in Bristol
- Improv Courses in Manchester
- Improv Courses in Sheffield
- Improv Courses in Leeds
- Improv Courses in Edinburgh
- Improv Courses in Leicester
- Improv Courses in Coventry
- Improv Courses in Bradford
- Improv Courses in Cardiff
- Improv Courses in Belfast
- Improv Courses in Nottingham