- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
114 Healthcare IT courses
CT14: Clinical Trial Safety Reporting Requirements in the EU and USA
By Zenosis
This course sets out the legal and regulatory requirements for safety reporting in clinical trials of medicinal products under the jurisdictions of the European Union and the USA. It builds on the foundation laid by our companion course CT13, Safety Reporting in Clinical Trials, and provides greater detail of specific requirements in those jurisdictions.

CT13: Safety Reporting in Clinical Trials
By Zenosis
This course explains the regulatory requirements for the reporting of adverse events and suspected adverse reactions in clinical trials. It describes how investigators should report to sponsors, and how sponsors should report to regulatory authorities and other stakeholders in the safety of investigational products. It explains how events are characterized as serious or non-serious, expected or unexpected, and it distinguishes the requirements for each category. It describes controlled vocabularies used for coding of events in reports.
CT11: How to Gain Authorisation for Clinical Research Under the EU Clinical Trials Regulation
By Zenosis
This course sets out the procedures that sponsors need to follow to gain authorisation to conduct clinical trials under the Regulation, and it summarises and links to the extensive guidance available from the European Commission and the European Medicines Agency. Its companion course CT12 sets out the procedures that sponsors need to follow to conduct authorised clinical trials in compliance with the Regulation. The two courses therefore provide an ideal foundation for understanding and complying with the new law.
48-Hour Knowledge Knockdown! Prices Reduced Like Never Before. Healthcare GDPR is an essential course for professionals in the healthcare industry. Learn how to effectively comply with UK data protection laws, maintain the privacy and security of patient information, and stay up-to-date on the latest trends in healthcare data protection. Discover how to build a robust healthcare GDPR strategy to protect sensitive information, minimise the risk of breaches, and ensure the privacy of patients. This Certificate in Healthcare GDPR Training at QLS Level 3 course is endorsed by The Quality Licence Scheme and accredited by CPDQS (with 120 CPD points) to make your skill development & career progression more accessible than ever! In health and social care organisations, there are more stringent regulations governing the collection, processing, and storage of personal data. Additionally, a healthcare GDPR training makes sure that a company efficiently protects patient information. You will learn the foundations of maintaining the integrity of healthcare data with this Healthcare GDPR course for the healthcare industry. Take our Healthcare GDPR course and get ready for: Understanding the General Data Protection Regulation (GDPR) and its implications for healthcare professionals Knowing how to handle personal data in accordance with GDPR regulations Being able to implement appropriate security measures to protect patient data Understanding the rights of patients under GDPR and how to respond to requests for access to their data Knowing how to handle data breaches and report them in accordance with GDPR requirements. Why Prefer This Healthcare GDPR at QLS Level 3 Course? Opportunity to earn a certificate endorsed by the Quality Licence Scheme & another accredited by CPDQS which is completely free. Get a free student ID card! (£10 postal charge will be applicable for international delivery) Innovative and engaging content. Free assessments 24/7 tutor support. Our Healthcare GDPR course is designed specifically for helping the learners to understand and comply with the General Data Protection Regulation (GDPR). Through interactive lessons and practical examples, you will learn about the key principles of GDPR and how they apply to the healthcare sector. You will also learn about the rights of patients under GDPR and how to handle their personal data in a compliant manner. Additionally, you will learn about the importance of implementing appropriate security measures to protect patient data, as well as how to handle and report data breaches. By the end of this Healthcare GDPR Training course, you will have the knowledge and skills needed to ensure compliance with GDPR in your healthcare practice. *** Course Curriculum *** Here is the curriculum breakdown of the Healthcare GDPR at QLS Level 3 course: Module 01: Introduction to GDPR Module 02: GDPR and Healthcare Setting Module 03: General Data Protection Regulations Explained Module 04: Lawful Basis for Preparation Module 05: Responsibilities and Obligations Module 06: Electronic Medical Records Module 07: Rights and Breaches Assessment Process You have to complete the assignment questions given at the end of the course and score a minimum of 60% to pass each exam. Our expert trainers will assess your assignment and give you feedback after you submit the assignment. You will be entitled to claim a certificate endorsed by the Quality Licence Scheme after you have completed the Certificate in Healthcare GDPR Training at QLS Level 3 exam. CPD 120 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Healthcare professionals, including doctors, nurses, and other healthcare staff Those working in healthcare administration or management roles Those responsible for handling patient data in a healthcare setting Those interested in understanding the legal requirements for handling personal data in the healthcare sector Those looking to ensure compliance with GDPR in their healthcare practice. Requirements No prior background or expertise is required. Career path Compliance Officer - Average Annual Income £35,686 Data Protection Officer (DPO) - Average Annual Income £44,557 Healthcare Administrator - Average Annual Income £23,526 Healthcare Consultant - Average Annual Income £42,500 Legal Professional - Average Annual Income £43712 Certificates Certificate in Healthcare GDPR Training at QLS Level 3 Hard copy certificate - Included After successfully completing the Certificate in Healthcare GDPR Training at QLS Level 3, you can order an original hardcopy certificate of achievement endorsed by the Quality Licence Scheme andalso you can order CPDQSAccredited Certificate that is recognised all over the UK and also internationally. The certificates will be home-delivered, completely free of cost. CPDQS Accredited Certificate Digital certificate - Included

ICT01: Compliance with Regulation 21 CFR Part 11 on Electronic Records and Electronic Signatures
By Zenosis
21CFR11 applies to records that are required to be submitted to the FDA, or that are subject to FDA inspection, and that are in electronic form – that is, as computer files. It applies to all computer systems used to create, modify, maintain, archive, retrieve, or transmit such records – from a humble spreadsheet program to a complex information management system.

SUB04: Preparing Submissions in the Common Technical Document (CTD) Format
By Zenosis
The CTD is the internationally recognised standard format for submissions to medicines regulatory authorities. In the European Economic Area, the USA and Canada, the CTD, in its electronic format (eCTD), is mandatory for all applications for marketing approval and all subsequent related submissions. The CTD is accepted in many other countries, being mandatory for new prescription medicines in some. This module explains the rationale for the CTD and provides guidance on its structure and format and the ways in which it is used.

PV04: Signal Detection and Management in Pharmacovigilance
By Zenosis
This module provides a guide to signal detection and management for approved products. The subject is presented as a process comprising four stages: signal detection, signal validation, signal analysis and prioritisation, and risk assessment and minimisation.

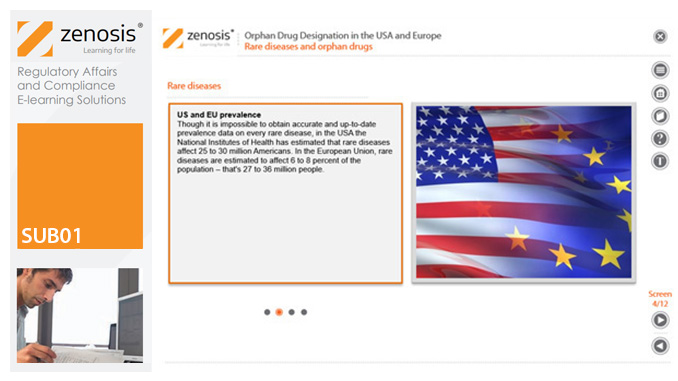
SUB01: Orphan Drug Designation in the USA and Europe
By Zenosis
Medicines for the prevention, diagnosis, or treatment of rare diseases have become known as ‘orphan drugs’ because of their commercial unattractiveness. Development of such products is successfully encouraged through incentives offered by regulatory authorities. To qualify for important incentives, the sponsor of a drug must gain ‘orphan designation’ for its use in an indication. This module describes the requirements for orphan designation and how to apply for it in the USA and the European Economic Area.
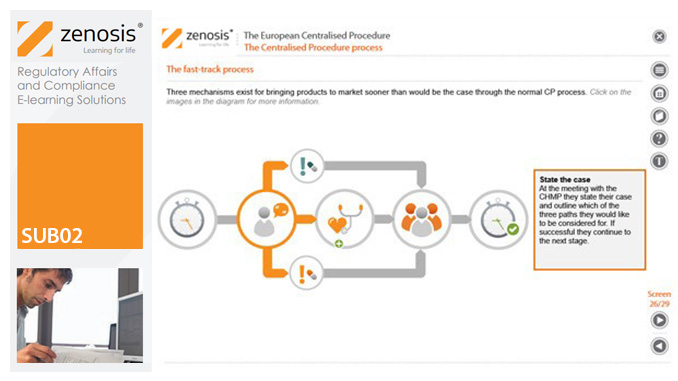
SUB02: The European Centralised Procedure (CP)
By Zenosis
The Centralised Procedure (CP) is one of three routes available to applicants to gain multinational marketing authorisation within the European Economic Area (EEA) on the basis of a single application. In the CP, one successful application leads to a marketing authorisation being issued by the European Commission that applies throughout the EEA. The CP is mandatory for certain types of products.
SUB12: Registration of Medicinal Products Based on Monoclonal Antibodies
By Zenosis
This module addresses characteristic issues influencing the registration of medicinal products based on monoclonal antibodies (mAbs), for use in humans. Regulatory requirements for the registration of biological medicinal products such as those based on mAbs differ in certain respects from those for small-molecule products. This is because of the distinct characteristics of biologics, such as complex structure and susceptibility to variation during manufacture.
Search By Location
- Healthcare IT Courses in London
- Healthcare IT Courses in Birmingham
- Healthcare IT Courses in Glasgow
- Healthcare IT Courses in Liverpool
- Healthcare IT Courses in Bristol
- Healthcare IT Courses in Manchester
- Healthcare IT Courses in Sheffield
- Healthcare IT Courses in Leeds
- Healthcare IT Courses in Edinburgh
- Healthcare IT Courses in Leicester
- Healthcare IT Courses in Coventry
- Healthcare IT Courses in Bradford
- Healthcare IT Courses in Cardiff
- Healthcare IT Courses in Belfast
- Healthcare IT Courses in Nottingham