- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
BOHS P904 - Management and control in leisure, display, therapy and other non-industrial systems Online
By Airborne Environmental Consultants Ltd
BOHS P904 - Management and control in leisure, display, therapy and other non-industrial systems is there to provide background and an overview of the risk of Legionella infection and how it can be controlled in leisure, display, therapy and other non-industrial water systems. It is a requirement of this course that candidates have successfully completed P901- Legionella- Management and Control of Building Hot and Cold Water Services [Syllabus GM.1]. Where both P901 and P904 courses are run on subsequent days or as a combined course then this pre-requirement is waived.

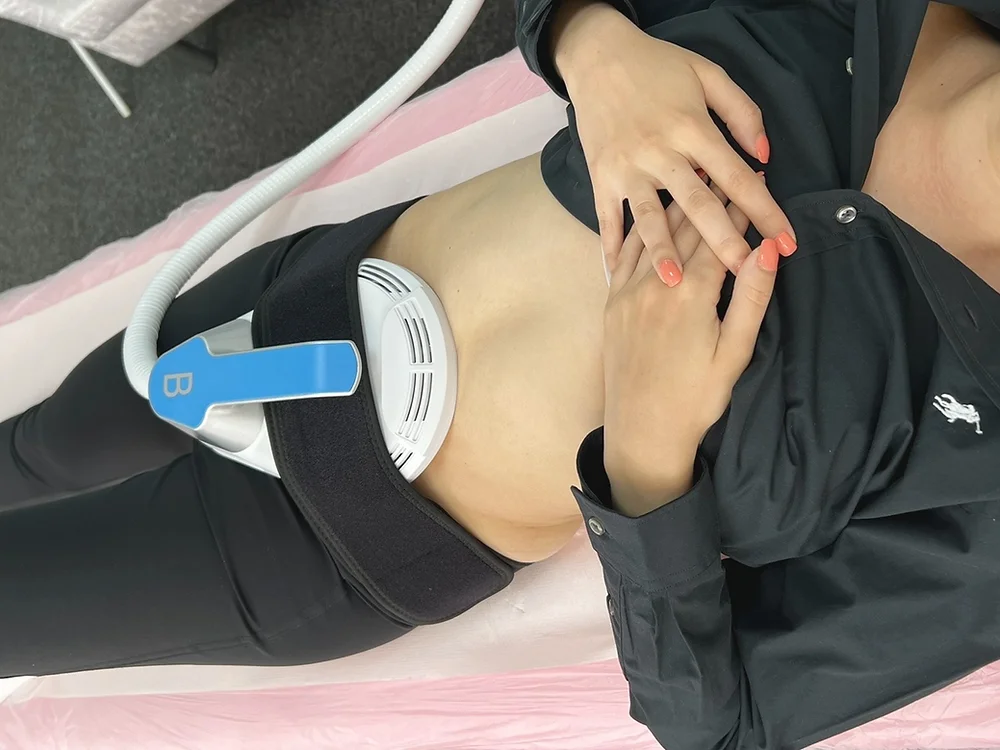
In person High-intensity electromagnetic Technology (HIEMT)/ EMS
By KBH Training Academy
High-intensity electromagnetic Technology (HIEMT)/ EMS What is High-intensity electromagnetic Technology (HIEMT)/ EMS? High-intensity electromagnetic technology (HIEMT) is an acronym for high-intensity electromagnetic technology. HIEMT contracts muscles in a specific location with a powerful but safe form of electromagnetic stimulation. These magnetic fields generate electrical currents, which are then transferred to the muscles, where they contract often enough to develop muscle and burn fat. Results, which can be as dramatic as looking to be equal to six months of dieting and workouts, COURSE CONTENT: * Health and safety * Sterilisation and disinfection * Appearance of the therapist * Ergonomics * Handwashing * Cellulite * Anatomy and physiology * Hiemt vs EMS * The consultation process * Machines explained * Treatment protocols * Aftercare TRAINING KIT: Please get in touch if you wish to purchase a machine HOW DOES THE COURSE WORKS? The course is divided into 2 parts, the first part is theoretical which you have to complete before you come for your practical training, and the second one is a practical assignment. The practical assignment is done on the day which will be agreed upon course purchase. You will spend around 2 hours practising on a model in our venue in London E106RA. We will call you to arrange a date once you sign up for the course. WILL I REQUIRE MODEL? Yes, usually 1 model is required ARE THERE ANY PREREQUISITES? We’re pleased to offer courses to people with lots of different experiences. However, previous experience nor qualifications are not necessary if you would like to enrol on our Course. CERTIFICATE: You will receive an end of course certificate which is accredited by the cpd group and allows you to work on public PAYMENT By paying for the course you agree to our Terms and Conditions
Aim To equip care staff with the knowledge, confidence, and practical skills to respond effectively and safely when a resident experiences a cardiac arrest, ensuring best practice in emergency resuscitation procedures. Learning Objectives By the end of the session, staff will be able to: Recognise the signs of cardiac arrest in a care home setting. Initiate a safe and effective emergency response, including correct positioning of the resident. Perform high-quality CPR on an adult using correct technique and rhythm. Use a defibrillator (AED) confidently and correctly. Identify environmental risks and adapt the environment to enable effective resuscitation. Demonstrate teamwork and role allocation in an emergency. Reflect on the importance of debriefing and support following a resuscitation incident. Location for delegates attending in person Foresters Nursing Home, Walton Pool, Stourbridge DY9 9RP Timings of the course 9.30am to 11.30am 12.00pm to 2.00pm 2.30pm to 4.30pm Before you book: Please do not send along staff who have just worked a nightshift. People arriving tired, stressed or late cannot gain the most benefit from a training course. We reserve the right to turn away individuals too exhausted to focus on the training or staff who turn up late for a course and disturb the flow of the proceedings. All CH Care Training courses are fully inclusive, but if you have any concerns about a member of staff's ability to participate because of physical, sensory or learning differences then please let us know in confidence in advance, so that we may make the appropriate adjustments. Cancellations CH Care Training operates a sliding scale of cancellation; If you cancel more than two weeks before the start date of a course, you can ask for a full refund, to change the names of the delegates being sent along, or to transfer a delegate to another training date. If you give less than two weeks but more than one week's notice of cancellation, 50 percent of the booking can be refunded. If you cancel less than a week from the training delivery date we wont be able to refund. There are no refunds for staff who do not turn up on the day of the training course. If CH Care Training needs to change the date or cancel a course for any reason we will give you the maximum notice we can. In exceptional circumstances we may cancel a course due to extreme weather or circumstances at the training venue. You will be offered a full refund or the opportunity to transfer your booking to an alternative date. Please ask your staff to read the following guidance before they attend the course: Arriving at the Venue The course will start promptly so please arrive at least 15 minutes before the start time of the training to allow plenty of time to use the toilet, and be seated ready for the start of the course. You can call Training direct on 0798 999 5180 if you are struggling to arrive on time. Please note that the meeting will be locked to late arrivals 10 minutes after the actual course starts. During the course As this is a working environment please ensure you allow the necessary time and focus to get the best from the material being presented. Please bring pen and paper to make notes during the course. Our training sessions are fun and very interactive. We will encourage plenty of comments, points of view and the sharing of lived experiences. Delegates should not share any sensitive information about a client or organisation. Our session agreement asks delegates to maintain confidentiality about individuals and organisations and to respect the views of others on the course. All CH Care Training courses are inclusive, but if you have any concerns about your ability to participate, such as sensory or learning differences then please let the Trainer know in confidence before the start of a course, so that they may make the appropriate adjustments. If the course contains practical elements such as Moving & Handling or First Aid, then please wear appropriate clothing to take part in these activities. You will be asked confidentially if you have any health concerns that could prevent you from taking part in the practical activities. Please let a Trainer know if you have any concerns about taking part in the practical courses. Refreshments are provided for free. Please bring a packed lunch for any full-day courses. After the course We will email you a pdf of the course once the training has finished and we have checked that everyone was able to join. If your Manager booked you onto the course using your work or personal email, or if you book yourself onto a course, you will receive the pdf directly. If your Manager booked you onto the course using your organisation’s email address a copy of the course will be available on request from your Manager. We will also email a link to an online evaluation and we ask that you please fill this in and return it to us so that we can continue to improve our services. All of our evaluations are gathered anonymously. If you have any questions, please contact Training on 0798 999 5180 or email sales@chcaretraining.co.uk.

Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

A fantastic opportunity to train in an ancient modality of treatment that science has now proven. You will be taught about the causes of disease, plant medicine, spiritual healing and Shamanic journeying. You will be encouraged to establish connections with your spirit guide helpers. There will be support for you in developing your own business and providing training for those you train. Lunch and refreshments all included. A deposit of £50 is taken to book your place and the balance of £250 to be paid when you arrive. Please contact us for more details, happy to answer any queries you may have.

We run Level 1 and Level 2 Reiki weekend Courses. The course starts at 9.00am on Saturday and will finish at approximately 4pm on Sunday. 1st Weekend - Level 1 Reiki, Developing the Healer Level 1 Reiki Practitioner Training Gathering wild plants for medicine What is the cause of diss-ease? Treating cause not symptoms Medicine wheel Reiki and treatments for self-healing, working in pairs Learning “Dai Chi” for daily empowerment Drumming into spirit world Reiki Level 1 Certification 2nd Weekend – Level 2 Reiki, Preparing the Practitioner 2nd Degree Reiki Practitioner Training Review of previous weekend course and spiritual awakening Working with patients, legalities, expectations, environment Foraging wild herbs and cultivated Using herb plants as food and medicine Using the nutritional systems profile questionnaire as a diagnostic tool Looking at body systems and healing them Medicine Wheel Drumming into spirit world Reiki Level 2 Certification A minimum of 6 months working with patients must be completed before doing your Reiki Master and Teacher Training. Both weekend courses includes all meals, refreshments and certification in both Level 1 and Level 2 Reiki Practitioner. The cost for Level 1 is £200.00 total with a £50.00 deposit to secure your place. The cost for Level 2 is £200.00 total with a £50.00 deposit to secure your place.

Search By Location
- Health Courses in London
- Health Courses in Birmingham
- Health Courses in Glasgow
- Health Courses in Liverpool
- Health Courses in Bristol
- Health Courses in Manchester
- Health Courses in Sheffield
- Health Courses in Leeds
- Health Courses in Edinburgh
- Health Courses in Leicester
- Health Courses in Coventry
- Health Courses in Bradford
- Health Courses in Cardiff
- Health Courses in Belfast
- Health Courses in Nottingham


