- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
34947 FA courses
ALLMI Lorry Loader -2 Day Novice Course | Milton Keynes | 1 - 2 August 2025
By Total Compliance
Equipment used - Lorry mounted cranes and Hook attachments. Who Would Do This Training? Anyone who operates or intends to be operating lorry mounted cranes (sometimes referred to as HIAB’s) should undertake training as is required by the Lifting Operations and Lifting Equipment Regulations 1998. This course is made to suit novice operators, and are designed to ensure the efficient and safe operation of equipment. HIAB is really a brand name and the correct term for the equipment is ‘lorry mounted crane’ or ‘lorry loader crane’ (when mounted to a commercial vehicle). Other manufacturers of these types of cranes include Atlas, Cormach, Fassi, HMF, Hyva, Palfinger, Penny Hydraulics and PM. Which Industries Train with ALLMI? The ALLMI Lorry Loader Course is recognised by CSCS. The Lorry Loader Crane courses and Slinger Signaller courses, certificated by ALLMI, cover a wide variety of hydraulic cranes, both manually and electronically operated. Manuals and Postage of ALLMI Certificate and Card are included in your Ticket Price

Licensed Facilitator Training - July 2025 Cohort
By Clare Martin
Private space to access recordings from the live training. For the viewing of the July 2025 Cohort only. Available for 6 months.

Overview Adult Nursing: Adult Nursing Course Online If you want to learn about Adult Nursing: Adult Nursing Course and become an expert in the field, you have arrived at the right place. Do you want to work in a field that involves assisting those in need? If you enjoy working with people and are interested in healthcare, this Adult Nursing: Adult Nursing Course is for you. This Adult Nursing: Adult Nursing Course will teach you the skills and techniques needed to work as a Nursing Assistant. The Adult Nursing: Adult Nursing Course covers advanced patient hygiene procedures, effective nursing communication, and ensuring patient safety and comfort in the adult care setting. Patient care safety measures teach the learner techniques for preventing falls and protecting patients at risk of injury, burns, or scalds, as well as steps to prevent infection spread in the care setting. You will also gain a thorough understanding of asepsis in nursing through this Adult Nursing: Adult Nursing Course. Main Course: Adult Nursing Training Free Courses: Course 01: Nursing & Prescribing Course 02: Safeguarding Level 3 [ Note: Free PDF certificate as soon as completing the Adult Nursing: Adult Nursing course] Description Adult Nursing: Adult Nursing Course Online This Adult Nursing: Adult Nursing Course consists of 07 modules. Assessment Method of Adult Nursing: Adult Nursing Course After completing Adult Nursing: Adult Nursing Course, you will get quizzes to assess your learning. You will do the later modules upon getting 60% marks on the quiz test. Apart from this, you do not need to sit for any other assessments. Certification of Adult Nursing: Adult Nursing Course After completing the Adult Nursing: Adult Nursing course, you can instantly download your certificate for FREE. Who is this course for? Adult Nursing: Adult Nursing Course Online This Adult Nursing: Adult Nursing Course is ideal for: Nursing Assistants Nursing Associates Nursing Home Manager Requirements Adult Nursing: Adult Nursing Course Online To enrol in this Adult Nursing: Adult Nursing Course, students must fulfil the following requirements: Adult Nursing: Good Command over English language is mandatory to enrol in our Adult Nursing Course. Adult Nursing: Be energetic and self-motivated to complete our Adult Nursing Course. Adult Nursing: Basic computer Skill is required to complete our Adult Nursing Course. Adult Nursing: If you want to enrol in our Adult Nursing Course, you must be at least 15 years old. Career path Adult Nursing: Adult Nursing Course Online Enrolling on this Adult Nursing: Adult Nursing Course can lead you to the following job opportunities.

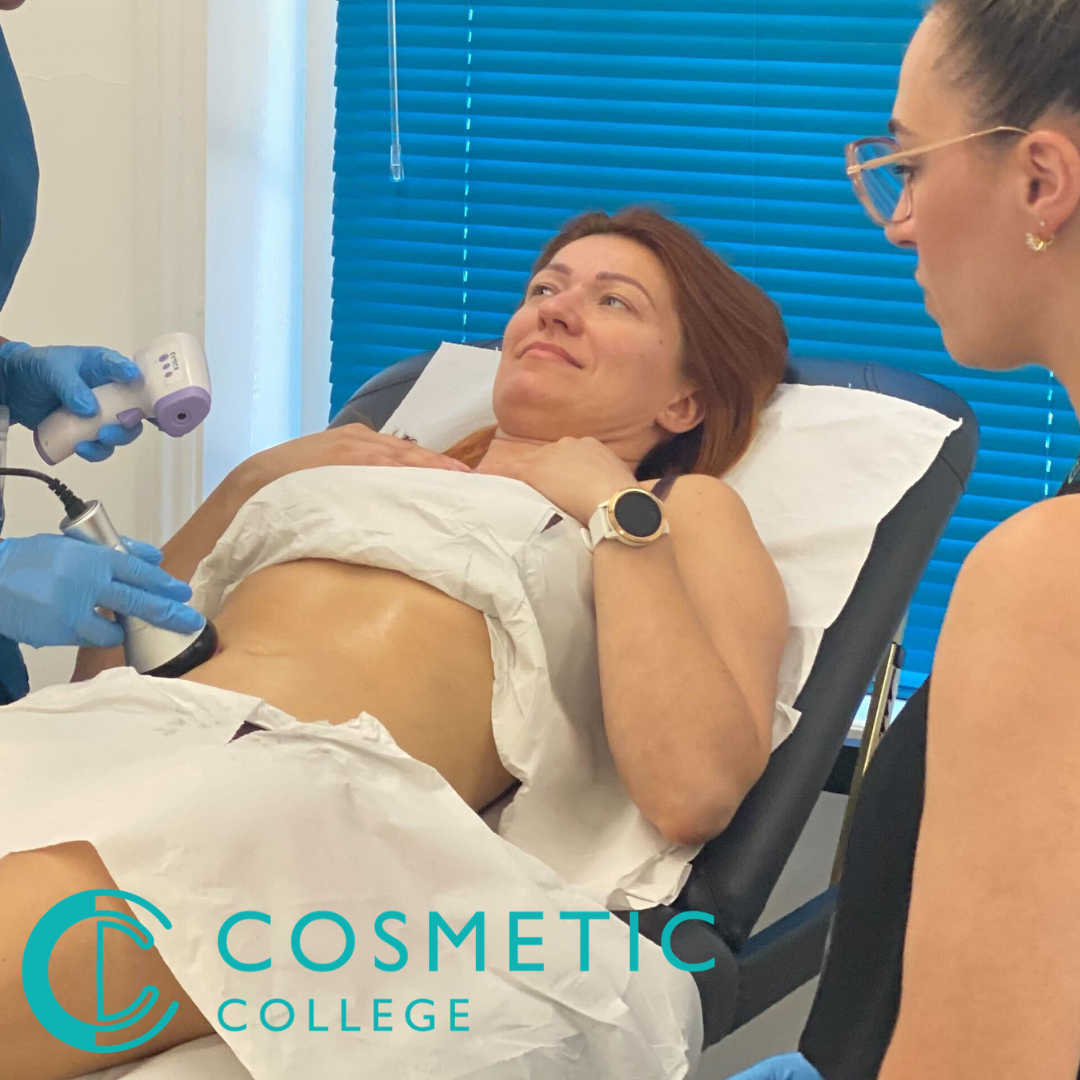
Body Contouring Training Package
By Cosmetic College
This course is designed to teach students how to safely and successfully carry out a variety of body contouring treatments at the best value available in the market. Our course offers a blend of practical training and online learning to give you the knowledge and skills to deliver face and body waxing services to your clients. Our courses are kept intimate with a maximum of 6 learners to a class. Courses Included Wood Therapy Lymphatic Drainage Massage Fat Freezing Ultrasound Cavitation Radio Frequency Skin Tightening This package is delivered in a combined format with e-learning provided to be completed ahead of an intense 4 day practical training days.
Safeguarding Children Level 3
By The Teachers Training
Advance your knowledge and skills in safeguarding children with our Level 3 course. Gain a comprehensive understanding of child protection policies, risk assessment techniques, and effective intervention strategies to ensure the safety and well-being of children in various settings.

Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

ALLMI Lorry Loader -2 Day Novice Course | Milton Keynes | 22 - 23 August 2025
By Total Compliance
Equipment used - Lorry mounted cranes and Hook attachments. Who Would Do This Training? Anyone who operates or intends to be operating lorry mounted cranes (sometimes referred to as HIAB’s) should undertake training as is required by the Lifting Operations and Lifting Equipment Regulations 1998. This course is made to suit novice operators, and are designed to ensure the efficient and safe operation of equipment. HIAB is really a brand name and the correct term for the equipment is ‘lorry mounted crane’ or ‘lorry loader crane’ (when mounted to a commercial vehicle). Other manufacturers of these types of cranes include Atlas, Cormach, Fassi, HMF, Hyva, Palfinger, Penny Hydraulics and PM. Which Industries Train with ALLMI? The ALLMI Lorry Loader Course is recognised by CSCS. The Lorry Loader Crane courses and Slinger Signaller courses, certificated by ALLMI, cover a wide variety of hydraulic cranes, both manually and electronically operated. Manuals and Postage of ALLMI Certificate and Card are included in your Ticket Price

ALLMI Lorry Loader -2 Day Novice Course | Milton Keynes | 27 - 28 June 2025
By Total Compliance
Equipment used - Lorry mounted cranes and Hook attachments. Who Would Do This Training? Anyone who operates or intends to be operating lorry mounted cranes (sometimes referred to as HIAB’s) should undertake training as is required by the Lifting Operations and Lifting Equipment Regulations 1998. This course is made to suit novice operators, and are designed to ensure the efficient and safe operation of equipment. HIAB is really a brand name and the correct term for the equipment is ‘lorry mounted crane’ or ‘lorry loader crane’ (when mounted to a commercial vehicle). Other manufacturers of these types of cranes include Atlas, Cormach, Fassi, HMF, Hyva, Palfinger, Penny Hydraulics and PM. Which Industries Train with ALLMI? The ALLMI Lorry Loader Course is recognised by CSCS. The Lorry Loader Crane courses and Slinger Signaller courses, certificated by ALLMI, cover a wide variety of hydraulic cranes, both manually and electronically operated. Manuals and Postage of ALLMI Certificate and Card are included in your Ticket Price

ALLMI Lorry Loader -2 Day Novice Course | Milton Keynes | 11 - 12 July 2025
By Total Compliance
Equipment used - Lorry mounted cranes and Hook attachments. Who Would Do This Training? Anyone who operates or intends to be operating lorry mounted cranes (sometimes referred to as HIAB’s) should undertake training as is required by the Lifting Operations and Lifting Equipment Regulations 1998. This course is made to suit novice operators, and are designed to ensure the efficient and safe operation of equipment. HIAB is really a brand name and the correct term for the equipment is ‘lorry mounted crane’ or ‘lorry loader crane’ (when mounted to a commercial vehicle). Other manufacturers of these types of cranes include Atlas, Cormach, Fassi, HMF, Hyva, Palfinger, Penny Hydraulics and PM. Which Industries Train with ALLMI? The ALLMI Lorry Loader Course is recognised by CSCS. The Lorry Loader Crane courses and Slinger Signaller courses, certificated by ALLMI, cover a wide variety of hydraulic cranes, both manually and electronically operated. Manuals and Postage of ALLMI Certificate and Card are included in your Ticket Price

Quality Assurance for Good Laboratory Practice
By Research Quality Association
Course Information A must-have programme for Quality Assurance auditors stepping into or honing their role within a Good Laboratory Practice (GLP) environment, this course offers invaluable, expert guidance for crafting a robust and efficient GLP audit programme. What will I learn? A solid regulatory foundation underpinning quality assurance activities Clarity on the roles of Quality Assurance, management, and study director within the framework of Good Laboratory Practice principles Enhanced efficacy in inspections and audits Heightened compliance with Good Laboratory Practice standards for your facility Unique insights into governmental monitoring activities within the GLP sphere. This course is structured to encourage delegates to Discuss and develop ideas Solve specific problems Examine particular aspects of GLP. Tutors Tutors will be comprised of (click the photos for biographies): Cate Ovington Director, The Knowlogy Group Ltd Jane Elliston Senior Quality Assurance Auditor, Battelle UK Shona Ross Head of QA, Tower Mains Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Welcome and Introductions 09:15 Good Laboratory Practice Standards and Regulations An insight into the background and history of Good Laboratory Practice. 09:45 Principles of Quality Assurance What is the role and responsibilities of QA in GLP. Maintaining the independence of QA and what is an audit. 10:30 Break 10:45 Standard Operating Procedures GLP requirements and QA involvement. 11:30 Study Plans GLP requirements and QA involvement. 12:05 QA Programme Risk based programme, what are study, process and facility audits. 13:00 Lunch 14:00 Inspections Attitudes, techniques and attributes. 14:40 Workshop 1 - Facility and Process Inspections An exercise in inspection planning and preparation for inspections. 15:15 Break 15:30 Workshop 1 - Feedback 15:45 The Auditor and Audit Conduct Attitudes, attributes and techniques. 16:30 Panel Session An opportunity for delegates to put questions to the panel of speakers. 17:15 Close of Day Day 2 09:00 Workshop 2 - A Mock Audit 10:45 Break 11:00 Workshop 2 - Feedback 11:30 Auditing the Study Report Techniques and methods for the QA audit of the study report. 12:00 Record Keeping and Data The impact of GLP on data and records management. 12:40 Lunch 13:25 Data Integrity A look at the OECD GLP guidance document; the expectations of the regulators and the involvement of QA - Where QA adds value. 14:15 Workshop 3 - Amendments to Study Plan and Deviations from the Plan What are they? What is the difference between them? How are they controlled? 15:00 Workshop 3 - Feedback 15:15 Break 15:30 Regulatory Compliance GLP Monitoring Authority monitoring for compliance with Good Laboratory Practice. 16:15 Panel Session An opportunity for delegates to put questions to the panel of speakers. 16:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Learn

Search By Location
- FA Courses in London
- FA Courses in Birmingham
- FA Courses in Glasgow
- FA Courses in Liverpool
- FA Courses in Bristol
- FA Courses in Manchester
- FA Courses in Sheffield
- FA Courses in Leeds
- FA Courses in Edinburgh
- FA Courses in Leicester
- FA Courses in Coventry
- FA Courses in Bradford
- FA Courses in Cardiff
- FA Courses in Belfast
- FA Courses in Nottingham