- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
785 Environment courses in Addlestone
Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Management of Portfolios (MoP) Practitioner: In-House Training
By IIL Europe Ltd
Management of Portfolios (MoP®) Practitioner: In-House Training In this MoP® Practitioner course, participants will have sufficient knowledge and understanding of how to apply and tailor the MoP guidance and to analyze portfolio data, documentation, and roles in relation to a given situation. MoP helps organizations ensure if the investments are done in the right, change initiatives, and implementing them correctly. This is achieved by: Prioritizing the programs and projects in terms of their contribution to the organization's strategic objectives and overall level of risk Managing the programs and projects consistently to ensure efficient and effective delivery Maximizing the benefit by providing the greatest return from the investment made What you will Learn Define the business case to get senior management approval for portfolio management Plan the implementation of portfolio management Select and adapt MoP principles, practices, and techniques to suit different organizational environments Evaluate examples of MoP information including documents and role descriptions Analyze the solutions adopted in relation to a given scenario Introduction Overview MoP Portfolio Definition Portfolio Management Roles Portfolio Management Documents MoP Practitioner Assignments Portfolio Management Documents Portfolio Delivery How to Implement Practice Exam MoP Practitioner Exam (taken online, after the class has ended)

Management of Risk (M_o_R) Foundation: In-House Training
By IIL Europe Ltd
Management of Risk (M_o_R®) Foundation: In-House Training This M_o_R® Foundation course prepares learners to demonstrate knowledge and comprehension of the four elements of the M_o_R framework: Principles, Approach, Processes, Embedding and Reviewing and how these elements support corporate governance. The M_o_R Foundation Course is also a prerequisite for the M_o_R Practitioner qualification. What you will Learn At the end of the M_o_R Foundation course, participants will gain competencies in and be able to: Describe the key characteristics of risk and the benefits of risk management List the eight M_o_R Principles List and describe the use of the key M_o_R Approach documents Create Probability and Impact scales Define and distinguish between risks and issues Create a Risk Register Create a Stakeholder map Identify the key roles in risk management Use the key techniques and describe specialisms in risk management Undertake the M_o_R Foundation examination Introduction Introduction to the M_o_R course What is a risk? What is risk management? Why is risk management so important? Basic risk definitions The development of knowledge about risk management Corporate governance and internal control Where and when should risk management be applied? M_o_R Principles The purpose of M_o_R principles Aligns with objectives Fits the context Engages stakeholders Provides clear guidance Informs decision-making Facilitates continual improvement Creates a supportive culture Achieves measurable value Risk management maturity models M_o_R Approach Relationship between the documents Risk management policy Risk management process guide Risk management strategy Risk register Issue register Risk response plan Risk improvement plan Risk communications plan M_o_R Process Common process barriers Identify contexts Identify the risks Assess estimate Assess evaluate Plan Implement Communication throughout the process M_o_R Perspectives Strategic perspective Program perspective Project perspective Operational perspective Risk Specialisms Business continuity management Incident and crisis management Health and Safety management Financial risk management Environmental risk management Reputational risk management Contract risk management

Fire Marshal / Fire Warden
By Prima Cura Training
The course covers the latest fire safety regulations and the roles and duties of employees and their responsibilities on Fire Safety. The course is designed to meet and comply with the Regulatory Reform (Fire Safety) Order 2005.

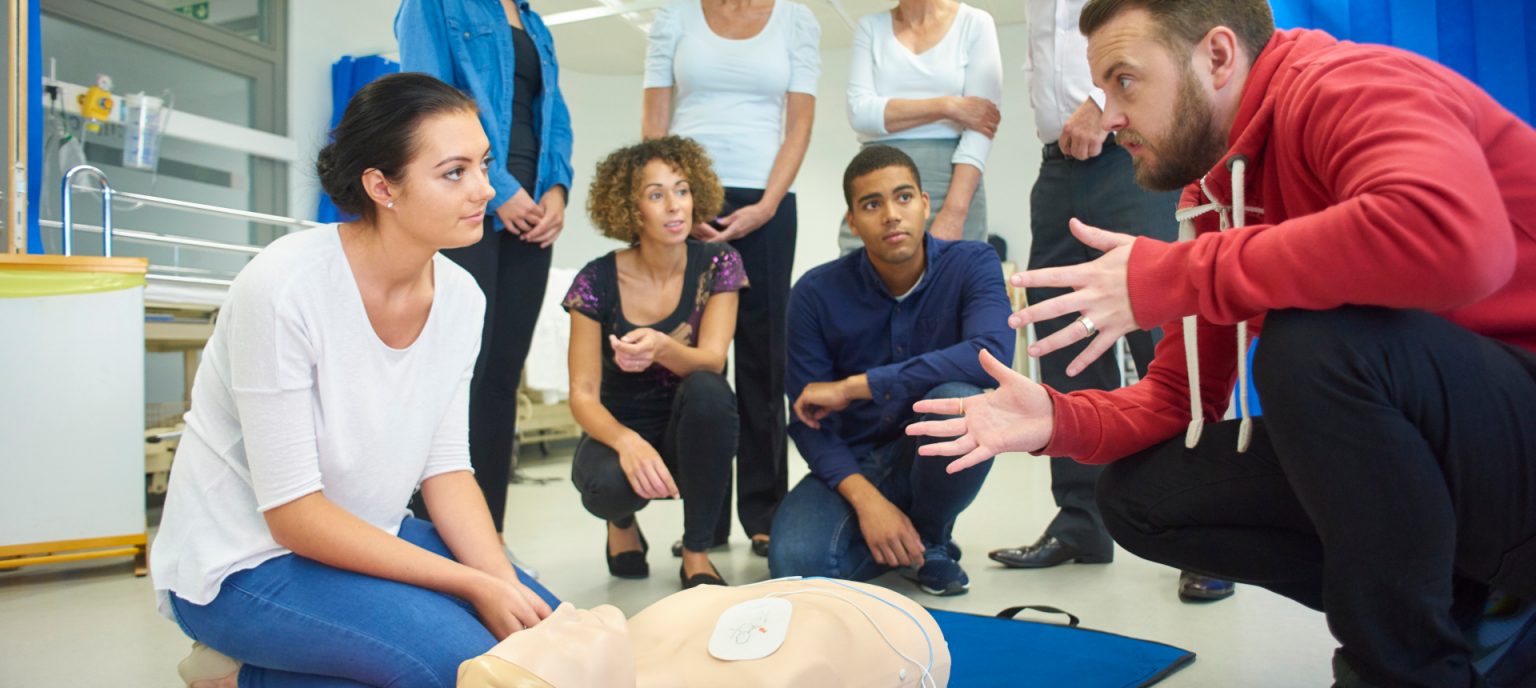
Emergency First Aid at Work (1-Day)
By Prima Cura Training
The Health and Safety (First Aid) Regulations 1981 Emergency First Aid at Work
Total SPB training course description A comprehensive look at Service Provider Bridging (SPB) as standardised in 802.1aq. This SPB course starts with the problems in traditional switched networks then moves onto to how SPB solves these problems. As IS-IS is key to loop free topologies in SPB networks, this protocols is also studied. What will you learn Explain the benefits of SPB. Explain how SPB works. Explain the role of IS-IS in SPB. Integrate SPB into existing networks. Total SPB training course details Who will benefit: Technical staff working with Ethernet. Prerequisites: Definitive Ethernet switching for engineers Duration 1 day Total SPB training course contents What is SPB? Layer 2 versus Layer 3, STP problems: One path, convergence, MAC explosion. Virtualisation issues: VRF. Service Provider Bridging (SPB), 802.1aq. Virtual Services Network. SPB variants 802.1Q, 802.1ad, Q-in-Q, VLAN IDs, 802.1ah, M-in-M, I-SID. SPBV, SPBM. SPBM frame format. How SPB works Node ID, Backbone Edge Bridges, Backbone MAC address, Customer MACs, I-SID. IS-IS. Forwarding database. VSN. IS-IS Link layer IS-IS, SPF trees, traffic management. IS-IS extensions for SPB. ISIS TLV. SPB services Data centre bridging, server virtualisation, multitenant applications. Integration of SPB into existing networks. VLAN to ISID mappings. Inter ISID routing, IP/SPB Layer 3 VRF extensions. Miscellaneous Multicast in SPB environments. Source specific multicast trees. SPB configuration. SPB deployment considerations.

Definitive SRv6 course description This one day SRv6 training course is a condensed, intensive program designed to provide network professionals with a fast-track introduction to SRv6 (Segment Routing over IPv6). SRv6 is a revolutionary networking technology that combines the power of IPv6 with the flexibility of Segment Routing, enabling efficient and scalable network operations. In just one day, participants will gain essential knowledge and practical skills to understand, configure, and work with SRv6 in modern network environments. Hands on sessions are used to reinforce the theory rather than teach specific manufacturer equipment. What will you learn Explain packet paths when implementing SLB. Explain how SRv6 works. Explain the difference between SR and SRv6. Implement SRv6. Troubleshoot SRv6. Definitive SRv6 course details Who will benefit: Network engineers, architects, and administrators who want to quickly grasp the fundamentals of SRv6 and its practical applications in their network. Prerequisites: Definitive Segment Routing for engineers Duration 1 day Definitive SRv6 course contents Introduction to SRv6 What is SRv6? Source based routing, difference between SR-MPLS and SRv6. IPv6 headers review, routing headers. SRv6 simplified solution. Hands on Enabling IPv6 in the legacy network. SRv6 transport Segment Routing Extension Header. SRv6 segment identifiers. End SID, End.X SID. ISIS distribution of SIDs. Header processing in a SRv6 topology. Locators. Hands on Configuring SRv6 transport. Analysing SRv6 operation. SRv6 services End.DT4 SID, End.DT6 SID. SRH encapsulation modes: Insert and Encap modes. SRv6 TE policy. Hands on Migrating to SRv6, TI-LFA protection. Micro loop avoidance. SRv6 integration with 'older' technologies MPLS. The role of iBGP and eBGP v6 sessions. Hands on Integration with legacy network. Troubleshooting SRv6 SRv6 ping and traceroute. Hands on: Used throughout the course during exercises.

Breathing Apparatus Awareness Training
By Vp ESS Training
The aim of this course is to give delegates who have to use full working breathing apparatus during their working activities a greater understanding of correct operational procedures. Book via our website @ ESS | Training Courses | Vp ESS (vp-ess.com) or via email at: esstrainingsales@vpplc.com or phone on: 0800 000 346

Equality, Diversity and Inclusion
By Prima Cura Training
Our Equality and Diversity Training course covers the Equality Act 2010 and discusses the details relating to discrimination as well as unfair treatment within a professional environment.

Business Process Modeling: In-House Training
By IIL Europe Ltd
Business Process Modeling: In-House Training This course is part of IIL's Business Analysis Certificate Program (BACP), a program designed to help prepare individuals pass the IIBA® Certification exam to become a Certified Business Analysis Professional (CBAP®). Learn more at www.iil.com/bacp A process model is a description of a process in terms of its steps or actions, the data flowing between them and participants in the process, machines, systems, and organizations involved. Modeling is a critical business analysis skill. It applies graphical and text communication techniques to describe the actions, objects, and relationships acted upon in the process and the steps that act upon them. This course teaches the technique of process modeling and ties together the core methods of process, behavior, and data modeling to enable business analysts to fully describe business processes in levels of detail from multiple perspectives. What you will Learn Upon completion, participants will be able to: Identify business processes and their components Work with UML diagrams Use process modeling in business diagramming Diagram and model business processes Foundation Concepts The role of the business analyst The IIBA® BABOK® Knowledge Areas Business Process Modeling (BPM) and the business analyst A practical approach to business process modeling The Context for Modeling Business Processes Overview of context for business process modeling Analyzing stakeholder information Modeling best practices Critical inputs for BPM: Business Rules Critical inputs for BPM: Context Diagrams Data Models Overview of data modeling Entity relationship diagrams Object-oriented approach Class diagrams Other data models Process Models - Part I (Non-UML) Overview of process modeling Data flow diagrams Workflow diagrams Flowcharts Process Models - Part II (UML) Overview of UML Process Models UML Activity Diagrams UML Sequence Diagrams Usage Models - Part I (Non-UML) Overview of usage modeling Prototyping options Static prototyping and storyboards Dynamic prototyping User Interface Design and user stories Usage Models - Part II (UML Use Cases) Overview of Use Cases Use Case diagrams Use Case descriptions Use Cases and the product life cycle Integrating the Models Overview of integrating the models General analysis best practices Specific analysis techniques summary Best practices for transition to design Summary and Next Steps What did we learn and how can we implement this in our work environments?

Search By Location
- Environment Courses in London
- Environment Courses in Birmingham
- Environment Courses in Glasgow
- Environment Courses in Liverpool
- Environment Courses in Bristol
- Environment Courses in Manchester
- Environment Courses in Sheffield
- Environment Courses in Leeds
- Environment Courses in Edinburgh
- Environment Courses in Leicester
- Environment Courses in Coventry
- Environment Courses in Bradford
- Environment Courses in Cardiff
- Environment Courses in Belfast
- Environment Courses in Nottingham