- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
98 Drug Safety courses delivered Online
PV07: Good Pharmacoepidemiology Practice
By Zenosis
Pharmacoepidemiology is the study of the use and effects of drugs in large numbers of people. It provides a bridge between clinical pharmacology and epidemiology. The increasing demand for real-world evidence of the safety, efficacy and utility of medicinal products has focused greater attention on pharmacoepidemiological research. This module will help those who plan and conduct such research, and analyse and report the findings, to follow good practice.

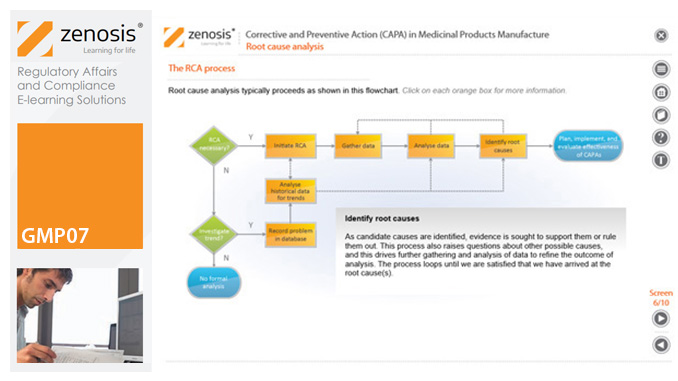
GMP07: Corrective and Preventive Action (CAPA) in Medicinal Products Manufacture
By Zenosis
A company’s Corrective and Preventive Action (CAPA ) system establishes how personnel should deal with manufacturing problems that have occurred or that may occur if not prevented. This module explains the principles of corrective and preventive action and describes typical CAPA procedure. It goes on to introduce root cause analysis and outline the role of progress tracking, escalating, and trending of CAPA procedures.
GMP06: Good Manufacturing Practice in Packaging Medicinal Products
By Zenosis
Packaging for medicinal products is subject to Good Manufacturing Practice rules similar to those for the products themselves. In this module we describe the functions that packaging must fulfil and the quality controls that are applied to packaging materials and operations. We set out the requirements for control of printed materials. We describe preparation, in-process control, and completion of a packaging run. Finally, we explain how to carry out reconciliation of packaging materials.

GMP02: Good Documentation Practice
By Zenosis
Good Manufacturing Practice (GMP) for medicinal products relies on documentation. Good Documentation Practice (GDocP) is that part of GMP that applies to the creation, maintenance, use, and retention of documents to provide assurance of the quality of products.
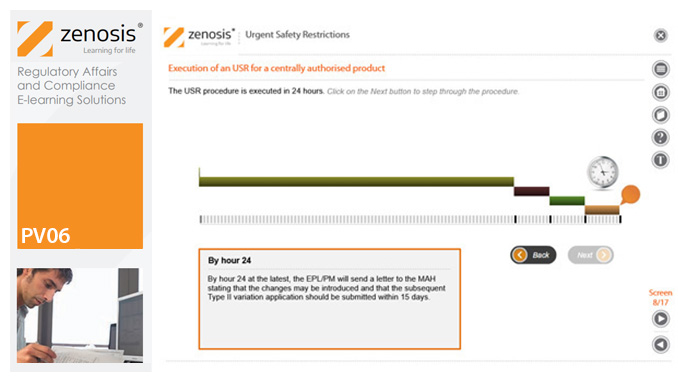
PV06: Urgent Safety Restrictions
By Zenosis
An Urgent Safety Restriction (USR) is a regulatory action taken, in response to a safety signal, to make an interim change to the terms of the marketing authorisation for a medicinal product in Europe. This module describes the principles and procedures for USRs.
GXP01- Good Practices (GxP) in Drug Development and Manufacturing
By Zenosis
This short entry-level module introduces the learner to good practices (GxP) in drug development and manufacturing. It outlines how the industry operates and how it is regulated. It identifies regulatory authorities and other important sources of guidance on Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), and Good Laboratory Practice (GLP).

GXP01: Good Practices (GxP) in Drug Development and Manufacturing
By Zenosis
This short entry-level module introduces the learner to good practices (GxP) in drug development and manufacturing. It outlines how the industry operates and how it is regulated. It identifies regulatory authorities and other important sources of guidance on Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), and Good Laboratory Practice (GLP).

Explore the intricate web of emotions, behaviors, and neurological processes underlying alcohol and drug addiction. Uncover the psychological mechanisms driving substance dependence and gain insights into effective intervention strategies. Delve into the complex terrain of addiction psychology for a comprehensive understanding of its impact on individuals and society.

GMP01b - Principles of GMP
By Zenosis
In this short course we present an overview of the main principles of GMP, and we outline some things that manufacturing personnel need to do to comply with requirements. We identify the principal goals of GMP as: prevention of contamination; prevention of mix-ups; scrupulous documentation; validation and maintenance of processes and equipment; quality assurance by an independent unit; and training. We place GMP in the context of a company’s quality management system.
CT04g - Data capture and management in clinical trials
By Zenosis
Capture and management of clinical trial data is a challenge. The industry is under pressure to obtain and analyse such data more quickly, while maintaining data integrity, so that products can be brought to market sooner. Effective planning and adequate resources can ensure clinical trials yield high quality data within strict timelines and budget requirements, at the same time satisfying regulatory standards. This short course describes the purpose of data capture and explores efficiencies in data management as part of the evolving regulatory landscape.