- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
5105 Development courses
Overview This sales coaching can offer sales those who square measure needed to create outgoing sales calls with a spread of verified skills, techniques and best practices to make sure additional sure-fire outcomes from a better proportion of the calls they create.

Overview The modern buyer is more knowledgeable and savvier than ever before. By taking a collaborative approach with the buyer and developing solutions, instead of relying on outdated sales tactics, professional sellers can create real value for clients and subsequently close more deals. Learn the solution selling method, and find out how to shift the emphasis from product features to the customer. By understanding how to implement the solution-selling methodology, you can create natural and pressure-less sales interactions that accelerate revenue growth and improve customer loyalty

Relationship Counselling in Mayfair: Transform Your Partnership with Our Comprehensive 3-Session Package Unlock the full potential of your relationship with our specialized Relationship Counselling in Mayfair. Our tailored package includes three intensive 1-hour sessions designed to strengthen your bond, enhance communication, and resolve conflicts effectively. During these sessions, couples will delve into essential areas such as: Communication Skills: Learn how to articulate your thoughts and feelings clearly and listen actively to your partner. Conflict Resolution: Discover strategies to manage disagreements constructively, ensuring both partners feel heard and respected. Emotional Intimacy: Enhance your emotional connection by understanding each other’s needs and fostering deeper empathy. Trust Building: Develop techniques to rebuild and maintain trust, a critical component of a healthy relationship. Stress Management: Learn how to handle external stressors together, creating a supportive and resilient partnership. Goal Setting: Align your personal and relationship goals to ensure a harmonious future together. Our experienced therapists in Mayfair are dedicated to providing a safe and supportive environment where couples can explore these vital aspects of their relationship. Each session is crafted to address your unique challenges and aspirations, ensuring personalized and effective guidance. Book your Relationship Counselling in Mayfair today and take the first step towards a stronger, more fulfilling relationship. Relationship Coaching London (relationshipsmdd.co.uk) Relationship counselling Mayfair⭐⭐⭐⭐⭐ | M.D.D Dating Coach, Couples Therapy, Breakup Counselling, Personal development Consultancy (relationshipsmdd.com)

Trap & Fault-Seal Analysis, Modeling for Oil & Gas and CO2
By EnergyEdge - Training for a Sustainable Energy Future
Dive deep into trap, fault, and seal analysis modeling for oil, gas, and CO2 with EnergyEdge course. Enroll in our classroom training today!

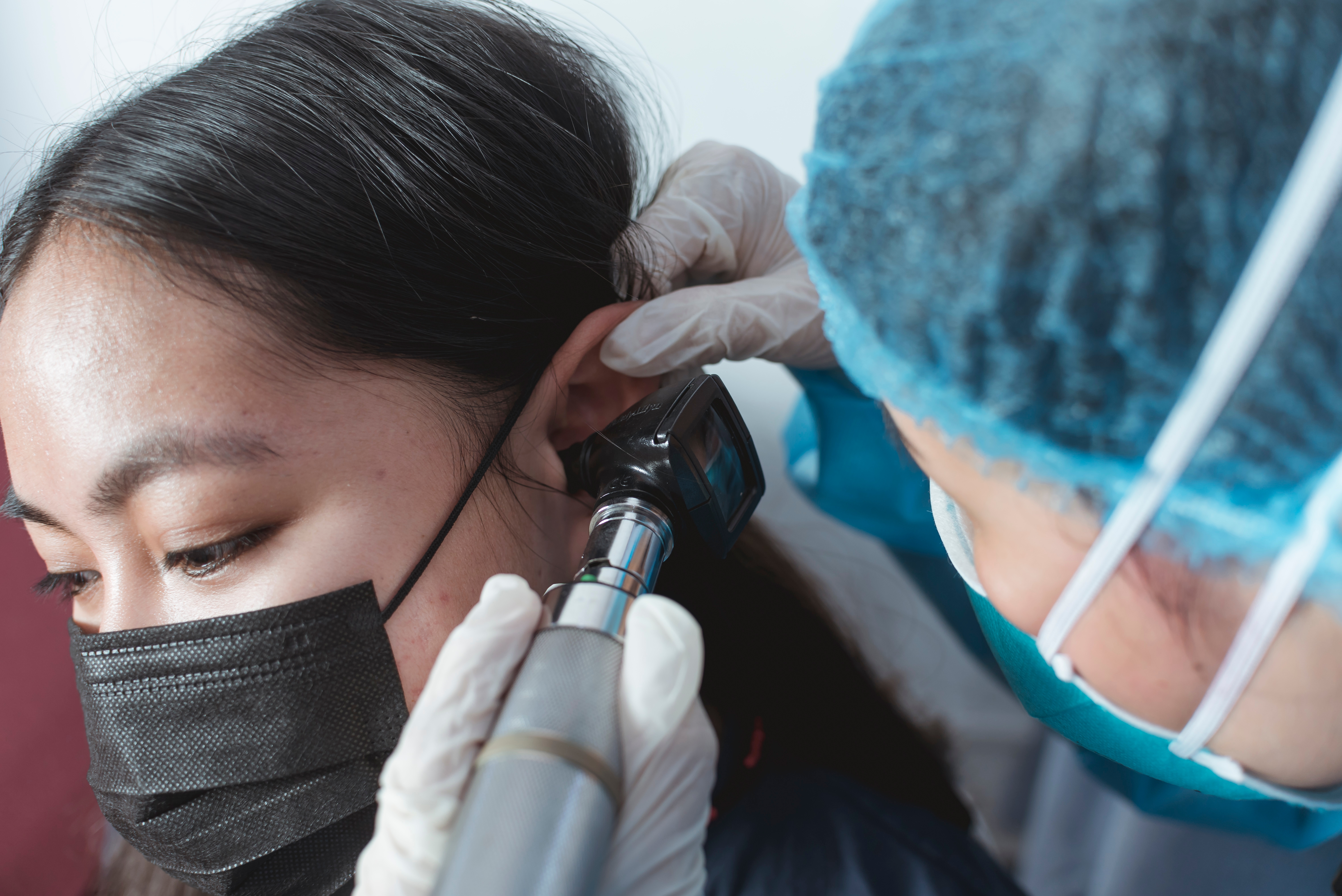
An Understanding of Ear Anatomy and Otoscopy
By Guardian Angels Training
Gain comprehensive knowledge of ear anatomy and otoscopy with our course. Ideal for healthcare professionals, medical students, and audiologists.
Overview This course examines the major tools, techniques, and principles aimed at improving quality and productivity in the public sector. To provide students with practical methods for improving public organizations, this course will cover accountability systems, performance measurement, pay-for-performance reward systems, contracting out, and organizational assessment

Large Scale Solar & Energy Storage - System Operations
By EnergyEdge - Training for a Sustainable Energy Future
About this Virtual Instructor Led Training (VILT) This 5 half-day Virtual Instructor Led Training (VILT) course will assist energy professionals in the planning and operation of a power system from renewable energy sources. The VILT course will discuss key operating requirements for an integrated, reliable and stable power system. The unique characteristics of renewable energy are discussed from a local, consumer centric and system perspective, bringing to life the ever-changing paradigm in delivering energy to customers. The course will explore the technical challenges associated with interconnecting and integrating hundreds of gigawatts of solar power onto the electricity grid in a safe and reliable way. With references to international case studies, the VILT course will also demonstrate the state of the art methodologies used in forecasting solar power. The flexibility of the invertor-based resources will facilitate higher penetrations of photovoltaic, battery electricity storage systems and demand response while co-optimizing customer resources. The contribution of inverter-based generators that provides voltage support, frequency response and regulation (droop response), reactive power and power quality with a high level of accuracy and fast response will be addressed. Furthermore, this VILT course will also describe how microgrids' controllers can allow for a fully automated energy management. Distributed energy resources are analyzed in detail from a technical and financial aspect and will address the best known cost based methodologies such as project financing and cost recovery. Training Objectives Upon completion of this VILT course, participants will be able to: Learn about renewable energy resources, their applications and methods of analysis of renewable energy issues. Review the operational flexibility of renewable energy at grid level, distribution network and grid edge devices. Understand and analyze energy performance from main renewable energy systems. Get equipped on the insights into forecasting models for solar energy. Predict solar generation from weather forecasts using machine learning. Explore operational aspects of a complex power system with variability from both the supply & demand sides. Manage the impact of the design of a Power Purchase Agreement (PPA) on the power system operation. Target Audience Engineers, planners and operations professionals from the following organizations: Energy aggregators who would like to understand the system operations of renewable energy power plants Renewable energy power system operator Energy regulatory agencies who aim to derive strategies and plans based on the feedback obtained from the power system operations Course Level Basic or Foundation Training Methods The VILT course will be delivered online in 5 half-day sessions comprising 4 hours per day, including time for lectures, discussion, quizzes and short classroom exercises. Course Duration: 5 half-day sessions, 4 hours per session (20 hours in total). Trainer Your first expert course leader is a Utility Executive with extensive global experience in power system operation and planning, energy markets, enterprise risk and regulatory oversight. She consults on energy markets integrating renewable resources from planning to operation. She led complex projects in operations and conducted long term planning studies to support planning and operational reliability standards. Specializing in Smart Grids, Operational flexibilities, Renewable generation, Reliability, Financial Engineering, Energy Markets and Power System Integration, she was recently engaged by the Inter-American Development Bank/MHI in Guyana. She was the Operations Expert in the regulatory assessment in Oman. She is a registered member of the Professional Engineers of Ontario, Canada. She is also a contributing member to the IEEE Standards Association, WG Blockchain P2418.5. With over 25 years with Ontario Power Generation (Revenue $1.2 Billion CAD, I/S 16 GW), she served as Canadian representative in CIGRE, committee member in NSERC (Natural Sciences and Engineering Research Council of Canada), and Senior Member IEEE and Elsevier since the 90ties. Our key expert chaired international conferences, lectured on several continents, published a book on Reliability and Security of Nuclear Power Plants, contributed to IEEE and PMAPS and published in the Ontario Journal for Public Policy, Canada. She delivered seminars organized by the Power Engineering Society, IEEE plus seminars to power companies worldwide, including Oman, Thailand, Saudi Arabia, Malaysia, Indonesia, Portugal, South Africa, Japan, Romania, and Guyana. Your second expert course leader is the co-founder and Director of Research at Xesto Inc. Xesto is a spatial computing AI startup based in Toronto, Canada and it has been voted as Toronto's Best Tech Startup 2019 and was named one of the top 10 'Canadian AI Startups to Watch' as well as one of 6th International finalists for the VW Siemens Startup Challenge, resulting in a partnership. His latest app Xesto-Fit demonstrates how advanced AI and machine learning is applied to the e-commerce industry, as a result of which Xesto has been recently featured in TechCrunch. He specializes in both applied and theoretical machine learning and has extensive experience in both industrial and academic research. He is specialized in Artificial Intelligence with multiple industrial applications. At Xesto, he leads projects that focus on applying cutting edge research at the intersection of spatial analysis, differential geometry, optimization of deep neural networks, and statistics to build scalable rigorous and real time performing systems that will change the way humans interact with technology. In addition, he is a Ph.D candidate in the Mathematics department at UofT, focusing on applied mathematics. His academic research interests are in applying advanced mathematical methods to the computational and statistical sciences. He earned a Bachelor's and MSc in Mathematics, both at the University of Toronto. Having presented at research seminars as well as instructing engineers on various levels, he has the ability to distill advanced theoretical concept to diverse audiences on all levels. In addition to research, our key expert is also an avid traveler and plays the violin. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information about post training coaching support and fees applicable for this. Accreditions And Affliations

Essentials of Recruitment and Selection
By Mpi Learning - Professional Learning And Development Provider
Getting the design and implementation of your recruitment and selection process right is the first critical step to attracting great people into your business.

Policy & Procedure Writing
By Mpi Learning - Professional Learning And Development Provider
All organizations have policies and procedures that guide how decisions are made and how the work is done in that organization. Professionally written policies and procedures increase organizational accountability and transparency and are fundamental to quality/standards assurance and quality improvement.

Course Description This training is designed for delegates who undertake a Healthcare Assistant role, or act as a support worker. You may also be training as a Lymphoedema Assistant.Please note price includes VAT Our fully inclusive HealthCare Assistant, Support Worker & Lymphoedema Assistant Training Programme Course Summary We know the role of the Health Care Assistant/Support Worker is integral to providing continued clinical care to people living with lymphoedema. As a key team member, the individual is trained at a high level to ensure best practice. This training is designed for delegates who undertake a Healthcare Assistant role, or act as a Support Worker, you may also be training as a Lymphoedema Assistant. Entry Requirements This training is suitable for: All Healthcare Assistants, Support Workers & Lymphoedema Assistants. Learning Format Your course is made up of a blended learning format meaning your content and modules will be taught through self-guided independent study and face to face clinical skills workshops. So, whether you're learning style is social, solitary, visual, kinesthetic, auditory or a combination we have a learning style to help you get the best out of the course. We hope that you make the most out of the tools that are available to you throughout the course but don't worry if you are not the most "tech-savvy", we have real people on hand to help you every step of the way. Assessment Criteria All students will undergo a series of course specific formative & summative assessments. Throughout the course you will be assessed through observation of practical tasks, written assessments, assignments, research and online tests. Course Contents On completion you will have a thorough understanding of Anatomy, Physiology and pathophysiology, allowing you to assess and treat lymphoedema with essential management skills of compression, movement and drainage, skincare, risk reduction and understanding and assisting with technical skills. An ongoing theme throughout the training will be our focus on treatment, health, wellness, and lifestyle skills. Training will include a deeper look at the lymphatic system and drainage pathways. Through our research using Lymphofluoroscopy Imaging (ICG), we will introduce you to Fluoroscopy Guided Manual Lymphatic Drainage (FG-MLD®) and the ‘Fill & Flush’ technique. On completion of this training, you will have the theory and skills to treat the patient with more complex and challenging lymphatic conditions, including the management of midline oedema ensuring we provide you with skills to get faster, long lasting results from your treatment. Course Inclusions As part of your training pack, LTA will provide all training support materials required to undertake the course. Such as but not limited to: LymphBalls™ Latest LTA Research & Development articles Lymphoedema supporting literature Access to in-house LTA training & demonstration videos Learning management platform (CANVAS) profile & course content Snacks & Refreshments throughout your practical skills workshops Dedicated support from the LTA training team Course Certification Our Lymphoedema Assistant Rehabilitation Programme will certify you as a ‘LTA Therapist’ on completion of both parts. The benefit of an LTA certification means you can; Write LTA Cert. after your name Display FG-MLD® Certified and LTA Cert. logo on your marketing material Be recognised through wearing you LTA cert. qualification badge Practice MLD to further enhance outcomes Be listed on our international directory of lymphoedema services Use an innovative technique and ensure effective treatment programmes Receive training and support from Jane Wigg & Team Access the latest developments in Lymphoedema care Have access to a private FG-MLD® Therapist FB Page for on-going support and advice. Recertification To maintain your LTA Therapist Certification, a 2-day recertification is required after 12 months after your initial training, and you will be required to attend a 1-day recertification every 2 years thereafter to maintain your certification of practice.

Search By Location
- Development Courses in London
- Development Courses in Birmingham
- Development Courses in Glasgow
- Development Courses in Liverpool
- Development Courses in Bristol
- Development Courses in Manchester
- Development Courses in Sheffield
- Development Courses in Leeds
- Development Courses in Edinburgh
- Development Courses in Leicester
- Development Courses in Coventry
- Development Courses in Bradford
- Development Courses in Cardiff
- Development Courses in Belfast
- Development Courses in Nottingham