- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
56650 Courses delivered Online
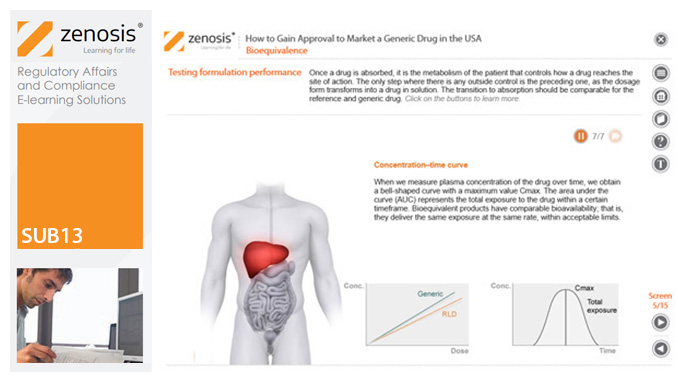
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
This short, step-by-step guide is designed to give all parents and those working with babies an introduction into the basic skills to help your baby if they have a medical emergency. This course covers the key elements of basic first aid for babies, for example choking, burns, how to put a baby into the recovery position and so much more. This course is not designed to replace a practical course but it will help empower you with skills and confidence to assess what is wrong and implement basic first aid to help. Prompt and appropriate First Aid reduces the pain and suffering experienced by your baby and could save their life. For our most comprehensive online course please visit our First Aid for Babies coursewhich won the Bizzie Baby Silver Award 2016. The course consists of illustrated step by step directions, flow charts, diagrams, videos and test yourself sections fully compatible with all computers and mobile devices. You will be able to stop and start as often as you like and print your Certificate on completion. You have continuous access to this course for 12 months. It is impossible to cover all eventualities within this course, or to equip you with the knowledge and skills to appropriately diagnose and treat in unpredictable real life situations. If you suspect serious illness or injury, you should always seek immediate professional medical advice. The author has made every effort to ensure the accuracy of the information contained within the course, however this course is merely a guide and the author does not accept any liability or responsibility for any inaccuracies or for any mistreatment or misdiagnosis of any person, however caused. The course material has been written by Emma Hammett, qualified nurse, first aid trainer and founder of First Aid for Life in conjunction with other medical and first aid professionals. If you have any queries concerning this course, please contact emma@firstaidforlife.org.uk Course contents: Action in an emergency Keep yourself safe Priorities of treatment What to put in your first aid kit Information to give the emergency services The primary survey – how to help in an emergency Danger Response Airway Breathing Unresponsive and breathing Recovery position How to put a baby into the recovery position Choking Burns Fitting/seizures/convulsions Meningitis Head injuries Final lesson

VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

SUB14: The Regulatory Pathway to Licensure of Follow-on Biologics (Biosimilars) in the USA
By Zenosis
The regulation of biological medicinal products is governed by different laws from those that apply to small-molecule synthetic drugs. Producing faithful copies of therapeutic proteins is more challenging than producing generic drugs. The US legal framework for the licensure of follow-on biologics, and accompanying regulatory guidance from the Food and Drug Administration (FDA), have been established only in recent years.

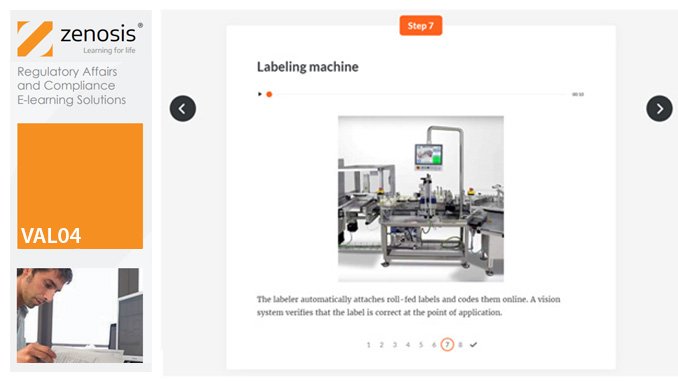
VAL04: Operational and Performance Qualification
By Zenosis
Having undergone Installation Qualification, before equipment can be used routinely in production, it needs to undergo Operational Qualification (OQ) and Performance Qualification (PQ). This module describes OQ and PQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
Introduction to Coaching - CPD Accredited
By Karen Blake Coaching
🚀 Introduction to Coaching Course: Unlock Your Coaching Potential! 🌟 Discover the art of coaching with our CPD Accredited 'Introduction to Coaching' course. 🎓 Gain insights into the GROW Model, motivation techniques, and advanced questioning skills. Ideal for aspiring coaches and professionals seeking career development.

Risk Management & Outsourcing School Excursions, Camps & Activities
By Xcursion
Risk Management & Outsourcing School Excursions, Camps & Activities Do you contract out school camps, excursions or international tours? If so, you need to understand what risks you've transferred & what risks you've retained. This course steps you through exactly what you need to know when contracting something out.

Give a compliment to your career and take it to the next level. This Logistics bundle will provide you with the essential knowledge to shine in your professional career. Whether you want to develop Logistics skills for your next job or elevate your skills for your next promotion, this Logistics bundle will help you stay ahead of the pack. Along with this Logistics course, you will get 19 premium courses, an original hardcopy, 20 PDF certificates (Main Course + Additional Courses) Student ID card as gifts. This Logistics Bundle Consists of the following Premium courses: Course 01: Logistic Management Course 02: Transport Management Diploma Course 03: Purchasing & Procurement Course - Level 3 Course 04: Commercial Law Course 05: Supply Chain Management Course 06: Material Management Course 07: Inventory Controller Course 08: Warehouse Safety Course 09: Delivery Driver Training Course 10: Driver Safety Awareness Certificate Course 11: Spill Management Training Course 12: Logistics of Crude Oil and Petroleum Products Course 13: Certificate in Anti Money Laundering (AML) Course 14: Process Improvement: Reduce Waste Course 15: Operation Management and Retailing Course 16: Retail Management - Level 5 Course 17: People Management Skills Level 3 Course 18: Bookkeeping Tool : Google Sheets Course 19: Paralegal Course 20: Time Management Enrol now in Logistics to advance your career, and use the premium study materials from Apex Learning. The Logistics bundle incorporates basic to advanced level skills to shed some light on your way and boost your career. Hence, you can strengthen your Logistics expertise and essential knowledge, which will assist you in reaching your goal. Moreover, you can learn Logistics from any place in your own time without travelling for classes. Logistics Course Curriculum Logistic Management Module 01: Introduction to Logistic Management Module 02: Planning Framework for Logistics Module 03: Supply Chain Management Module 04: Logistics Management and Organisation Module 05: Sourcing, Purchasing, and Procurement Module 06: Channels of Distribution Module 07: Supplier Relationship Management Module 08: Inventory Planning and Management Module 09: Principles of Warehousing Module 10: Transport Management Module 11: Negotiation Techniques Module 12: Customer Service and Logistics Certificate: PDF Certificate: Free (Previously it was £6*11 = £66) Hard Copy Certificate: Free (For The Logistic Management Course: Previously it was £10) CPD 200 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone from any background can enrol in this Logistics bundle. Requirements This Logistics course has been designed to be fully compatible with tablets and smartphones. Career path Having Logistics expertise will increase the value of your CV and open you up to multiple job sectors. Certificates Certificate of completion Digital certificate - Included Certificate of completion Hard copy certificate - Included You will get the Hard Copy certificate for the title course Logistic Management absolutely Free! Other Hard Copy certificates are available for £10 each. Please Note: The delivery charge inside the UK is £3.99, and the international students must pay a £9.99 shipping cost.
