- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
295 Courses delivered Online
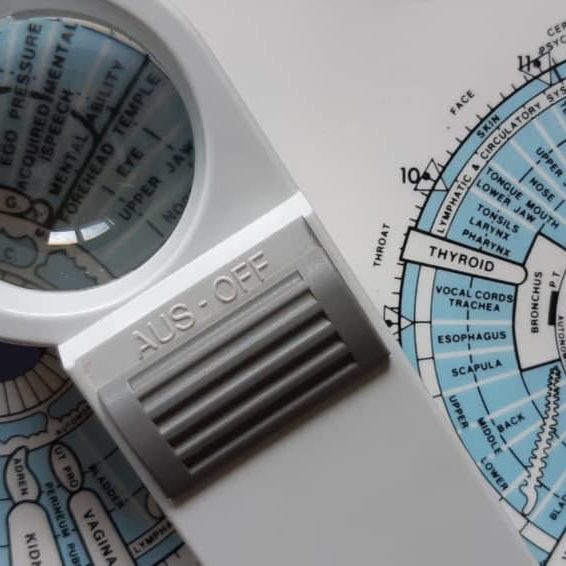
Iridology Diploma
By Plaskett International
LEARN HOW IRIDOLOGY CAN MAKE A HUGE CONTRIBUTION TO ANY COMPLEMENTARY PRACTICE A MESSAGE FROM THE AUTHOR I want to welcome you most warmly to the study of Iridology. Students of our course have taken their knowledge out into the world of practice and they have been able to see more penetratingly into the health of their patients. They have seen many truths about causes and effects in health and disease - that is what allows you to understand those extra things that make you into an even better healer. I think you are going to find this the most intriguing and absorbing study and, certainly, that is my sincere hope. As you precede, much of what you learn will amaze you and inspire wonder at the ways of the human body and mind. As you tread this very special road, I pass on to you the words that Bernard Jensen gave me years ago when I was his student, inscribed upon the inside cover of his book: “Seek the Higher Values in Life”. DR. LAWRENCE PLASKETT WHAT IS IRIDOLOGY? Iridology is the art of iris analysis. The iris is connected to the brain via the hypothalamus and can give naturopathic read outs on tissue conditions in various parts of the body. With training and practice it is possible to read signs indicative of biochemical, emotional and environmental influences that are hard to determine by other means. We can thus interpret health (and even aspects of personality) by close examination of the eyes, using suitable illumination and a magnifying glass. The close relationship between naturopathic iridology as an assessment tool and nutritional therapy and other naturopathic disciplines continues and grows closer. Now Iridology can make a huge contribution to complementary therapeutic practice and enhanced by our wonderful digital collection of eye photographs, the learning process with the Plaskett International College is a profound and exciting one. We teach Iridology quite separately from other topics and anyone who possesses, or expects to possess, a practitioner's qualification in any therapeutic discipline, may join the course. Course Duration 12 months Study Hours 200 hours Course Content 9 sections Course Fee £495 How Can Iridology Help Practitioners? Examples of how iridology can help practitioners Did you know that some iris features are so very plain that you can see them with the naked eye in ordinary social contact? You can see from two or three feet away in many cases that the person has a toxic digestive system (a strong wide dark ring around the pupil margin). You can often tell that the person has an overactive stomach (a narrow bright white ring very close to the pupil). You can tell when the skin is overlaid with toxins so that the skin's function in excreting toxins from the body is jeopardised (very narrow dark ring around the iris margin). You can tell in some people (rather advanced cases) that they suffer badly from sodium and potassium imbalance and have placed themselves at potential risk from cholesterol accumulation (the so-called corneal arcus, a white or off-white cloudy deposit, usually fairly thick, around the iris margin). Another example is the ring of spots or 'tophi' represented by the lymphatic rosary. Its mere presence tells one that there is sluggishness in the lymphatic system. When these tophi are darkly pigmented, the situation gives rise to concern for the possible generation of lymphatic illness. Using the precise positioning of iris reflex areas contained on the iris chart, one may distinguish many key points of analysis. Areas of stress and tension can be pinpointed by identifying 'contraction furrows’. Past injuries and adhesions show themselves as contortions of the normally regular and even iris fibres. You can answer questions like:- Is it the pancreas or the liver that is responsible for the trouble? Is the patient's hypertension caused by a defect of or toxic deposits in the particular brain area that is geared to control blood pressure? One of Jensen's rather dramatic illustrations is of the iris of a man who has just been shot. It shows the precise areas of tissue damage within the body and the response is very fast. The number of potential examples is almost without limit. The above may suffice to show the types of things that iridology can do for practitioners. We hope it will help you decide to study Iridology with the Plaskett International College. Course Overview The course covers the nature of iris observation, the nutritive zone, the iris chart, the chronic and acute, the intestinal and stomach zones and nerve collarette, the constitution type, respiratory system, lacunae, open lacuna, inherent weaknesses, the organs of elimination, other organs, special signs, complete diagnosis of a subject. The treatment of the topic follows the principles of Bernard Jensen in the USA. Once the basics have been learnt, the course teachings then extend considerably by bringing in the work of Dorothy Hall and of Dr Josef Deck, both of which are the subject of a special presentation during the course. The published insights of Farida Sharan and Harri Wolf, while not separately presented, also influence the presentation of the course material. Both the Australian School, (Dorothy Hall) and the German School, (Dr Deck/Harri Wolf), offer an added dimension to the study and interpretation of the constitution. PERSONALITY ASPECTS & CONSTITUTIONAL TYPES The study focuses upon the different personality aspects, which show up in different constitutional types. Dorothy Hall gives insights into what contributes to various different types of personality and their emotional and mental responses and their pre-dispositions to health or disease. Different sorts of people can have different priorities, preferences and imperatives built into their very nature from or before birth, sometimes determining the course of their entire lives and their attitudes to the world and to other people. AN EMPATHY BETWEEN PATIENT & PRACTITIONER The course teaches an understanding of these types and facilitates an empathy between patient and practitioner. It shows how people of the differing constitutional types are likely to go out of balance either mentally or emotionally and how their vulnerability to various physical ailments varies. The German School offers a very exciting and precise approach to the constitutional types, which is really quite different, but no less helpful. It highlights variations in the susceptibility to diseases of different organs and systems. THE 3 SCHOOLS OF THOUGHT It is a prime purpose of this course, not only to teach these differing positions, but also to demonstrate how it is that all three of these major schools of Iridology embody different aspects of the truth, how each is individually valuable and how a full and deep understanding of the meaning of 'constitution' can be gained through a sympathetic synthesis of the contributions from all three of these schools. BREAKDOWN OF THE COURSE SECTIONS In total there are 9 sections comprising of text, videos and iris images to study: SECTION 1 GENERAL PRACTICE AND AN ACCOUNT OF THE NUTRITIVE ZONE Areas Covered Iris colour Information that iridology can give us The structure of the eye and the iris Using the iris as an assessment tool The principle of reflex areas The Nutritive Zone Abnormality in the colon The Collarette (autonomic nerve wreath or anw) Diagnosis of the constitution based upon fibre structure Studies on images of real eyes SECTION 2 FEATURES OF THE FIBRES OUTSIDE THE COLLARETTE Areas Covered The general layout of fibres outside the collarette Inherent weaknesses First stage in further deterioration of an inherent weakness The meaning of darkness in the iris The development of discrete – open lacunae Lacunae Further notes about lightness and darkness amongst the fibres Healing lines Crypts Round the iris chart – the left iris Round the iris chart – the right iris Checking which structures and inside and which outside the collarette The organ systems The neural arc reflex SECTION 3 SPECIAL SIGNS Areas covered The corneal arcus (sodium ring, cholesterol ring, lipemic ring) The tophi (also lymphatic tophi or lymphatic rosary) Corneal Arcus The anaemia sign The catarrhal sign Acidity Grey background Scurf rim Circulatory ring Sphincter muscle (also called pupillary sphincter) Pigments (topastible or topolabile) Psoric spots Contrcation furrows Radial furrows SECTION 4 THE CONSTITUTIONS IN RELATION TO PERSONALITY TYPE AND DISEASE DISPOSITION Areas covered Very resilient Resilient average Moderately resilient Mildly resilient SECTION 5 MORE ABOUT WHITE SIGNS Areas covered Revision of distinctions between the different white signs Pictures of irises with white signs, with commentaries Further interpretation of the corneal arcus Further interpretation of the lytophi More general interpretation of the colour white SECTION 6 COLOURS IN THE IRIS AND OTHER SPECIAL SIGNS Areas Covered Yellow pigment in the iris Orange pigment Brown pigment Contraction furrows Radial furrows Psoric spots Pupillary border The “friendly fibrils” sign Summary of remedies SECTION 7 THE CONSTITUTION AND SIGNS ACCORDING TO THE GERMAN SCHOOL Areas Covered The German school of iridology Our approach to teaching the German school Introduction to the German constitutional types The lymphatic constitutions Mixed biliary constitution or biliary constitution Haematogenic (or haematogenous) constitution The way to use information on the German constitutions New signs that are specific to the German school Treatment recommendations for constitutional types SECTION 8 ADVANCED STUDIES OF THE IRIS Areas Covered Further details of the iris chart – its layout and its implications Neural arc reflex Deformation of pupil shape and position Advanced study of fibre separations, sinuosity, injuries & adhesions Lacunae of different shape and appearance The b3 bulge and the pterygium Working with genetically brown eyes SECTION 9 THE CONSULTATION & THE PRACTICALITIES Areas Covered Diagnosing pathology of individual critical organs Personality interpretations based upon the German school Conducting an iridology consultation Practical aspects of iris examination Making drawings of the iris and recording the data The uses, advantages and limitations of iris photography and its place in iridology practice Equipments and techniques of iris photography Using the computer to store and process digital images The interaction of signs Interpreting the whole iris in conjunction with the case study Pointers to treatment Carrying out case histories TESTIMONIALS Here's what students have to say about the course Emma Rubio, Health Coach Spain "As a Health Coach I decided to pursue my studies with the Plaskett College to become a Nutritional Therapist. For that, I am also studying Iridology. I am happy to have a tutor to answer my doubts and I like the flexibility that the College offers me. I love the subject of Iridology and the way it is explained, I also like having some videos of Dr Plaskett teaching Iridology as I admire him." Dr Ezequiel Lafontaine, Iridologist Puerto Rico "I LOVE IRIDOLOGY. I have over 30 iridology books, Italian, French, German, Spanish and English, plus over 4,000 photos from my own practice. I took this course for a refresher course and found the material was second to none." Mrs D. Moothy, Nutritional Therapist Mauritius “The distance learning courses have given me the opportunity to pursue my dreams through a program that was not only flexible and convenient for my schedule, but was also challenging and rewarding. I thoroughly enjoyed the readings and the assignments but most importantly, I enjoyed being able to do things at my pace. I must say that the most exciting and challenging course was the Iridology Diploma, and I am happy that I was able to do well in all the courses."
CT03: ICH Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data. The International Council for Harmonisation’s guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice.

ICA Certificate in Managing Sanctions Risk
By International Compliance Association
ICA Certificate in Managing Sanctions Risk Sanctions can be complex, and dealing with sanctioned parties can be risky. National or international sanctions may be issued against individuals, entities, groups or nations; or even trading activities/particular sectors. Those bodies charged with enforcing sanctions compliance are particularly active at the moment, with multiple fines for firms in recent years stretching into billions of dollars. Suitable for those working in financial crime and regulatory compliance, this could help you: understand sanctions and the international context discover the screening systems and controls define a sanctions governance framework manage alert investigations learn the challenges of change and the cost of getting it wrong This course is awarded in association with Alliance Manchester Business School, the University of Manchester. There are many benefits of studying with ICA: Flexible learning solutions that are suited to you Our learner-centric approach means that you will gain relevant practical and academic skills and knowledge that can be used in your current role Improve your career options by undertaking a globally recognised qualification that hiring managers look for as part of their hiring criteria Many students have stated that they have received a promotion and/or pay rise as a direct result of gaining their qualification The qualifications ensure that you are enabled to develop strategies to help manage and prevent risk within your firm, thus making you an invaluable asset within the current climate Students who successfully complete the course will be awarded the ICA Certificate in Managing Sanctions Risk and will be entitled to use the designation- Spec.Cert(Sanctions) This qualification is awarded in association with Alliance Manchester Business School, the University of Manchester. This course provides Participants with a detailed understanding of the following topics: Understanding sanctions The international context Defining a sanctions governance framework Sanctions lists and screening Managing alert investigations The cost of getting it wrong The challenges of change

Paralegal Complete Bundle - QLS Endorsed
By Imperial Academy
10 QLS Endorsed Courses for Paralegal | 10 Endorsed Certificates Included | Life Time Access

Description FCA Compliance Essentials Begin your journey in compliance management with practical knowledge of the laws and legislations that make up the regulatory field in the United Kingdom. Do you know that FCA sets an annual CPD requirement both for senior managers and employees of financial service firms? As per TC 2.1.15 & 2.1.16, the requirement is 35 hours in each 12 months, including 21 hours of structured CPD activities. Furthermore, TC 2.1.20G27/05/2022 defines structures CPD activities as follows: 'Examples of structured continuing professional development activities include participating in courses, seminars, lectures, conferences, workshops, web-based seminars or e-learning.' Our FCA Compliance Essentials Bundle, with an overall duration of about 40 hours, fulfils and even exceeds the FCA requirement in terms of CPD hours and is ideal for any professional within Financial Services. Enroll now and enjoy a special price! Who should attend? Banks' managers/officers Investment Services Companies managers/officers Insurance Companies managers/officers Listed Companies managers IT managers/officers of companies developing IT systems/applications for financial institutions in order for them to meet the regulatory requirements Lawyers Officers exercising control activities (internal auditors, inspectors, external auditors, operational risk managers etc) Professionals wishing to work in Compliance in the future Graduate or post graduate students. Professionals that wish to make an international career in Compliance and would like to have a broader picture of how Compliance works and its methodologies, at international level. Compliance Officers Risk Managers MLROs

Developing women academic leaders: coaching workshop programme for women/identify as women academics higher education aspiring to leadership roles - series 1 2023/24
By Coach Academic
This coaching programme is for aspiring women leaders in higher education, delivered in real time, fully online. Five workshops have been designed to support you in a group environment to work actively towards realising your professional goals, whatever they may look like, focused on leadership at all levels in higher education. HE needs leaders like you - workshops with Coach Academic will help to propel you to becoming one. For all academics who are female/identify as female. During this coaching experience you will explore and learn from: - your values and beliefs, and how they impact on your professional identity and actions - your beliefs about higher education, leadership, and yourself - your strengths and how to harness them for impact - your areas for development, and embracing the actions you choose to address these - your internal and external blockers, and how to manage these - your motivation - why do you want to lead in HE? From these you will be able to build your confidence and your leadership identity, and take concrete steps towards becoming the leader you aspire to be.

Developing women academic leaders: coaching workshop programme for women/identify as women academics higher education aspiring to leadership roles - series 2 2023/24
By Coach Academic
This coaching programme is for aspiring women leaders in higher education, delivered in real time, fully online. Five workshops have been designed to support you in a group environment to work actively towards realising your professional goals, whatever they may look like, focused on leadership at all levels in higher education. HE needs leaders like you - workshops with Coach Academic will help to propel you to becoming one. For all academics who are female/identify as female. During this coaching experience you will explore and learn from: - your values and beliefs, and how they impact on your professional identity and actions - your beliefs about higher education, leadership, and yourself - your strengths and how to harness them for impact - your areas for development, and embracing the actions you choose to address these - your internal and external blockers, and how to manage these - your motivation - why do you want to lead in HE? From these you will be able to build your confidence and your leadership identity, and take concrete steps towards becoming the leader you aspire to be.

Import/Export, Supply Chain Management and Purchase Ledger
By Imperial Academy
3 QLS Endorsed Diploma | QLS Hard Copy Certificate Included | 10 CPD Courses | Lifetime Access | 24/7 Tutor Support

Sales Training
By KEMP CENTER
Make your life easier, learn how to communicate effectively Do you want to be a good communicator? Professional training for salespeople complete with a certificate Learn how to enter into contracts and how to bookkeep including accounting for travel. Learn from the best in the industry An employment law specialist with extensive experience will be leading the course. Test your knowledge Take the tests and practical tasks in the course to consolidate your knowledge and skills. Take the most effective and comprehensive sales training on the Polish Internet. Join the best salespeople in the business and rocket the sales in your company. In this training discover how the most professional salespeople work every day. Learn how they attract their customers, make business connections, arrange meetings, have key conversations and most importantly how they finalize deals and close contracts. You’ll discover all of this and more with concrete real life examples from a TOP salesperson and manager with 12 years of experience in professional sales. By taking this course you will have instant access to the practices of one of the best salespeople in the business who will also show you their sales process from A to Z as well as interviews with real clients. The sales training is done through the National Education Center’s interactive platform and includes invaluable resources such as a hybrid workshop with practical tasks and training for salespeople complete with expert feedback. Parts of the course will require active participation in order to complete the training. You can take the course at your own pace from the comfort of your own home and will have access to the materials on the interactive platform for two years after completing the course Join the communication course and get the benefits: Learning from a professional instructor24/7 access from any devicePractical exercisesTests, quizzes and recapsCertificate of completionExtra materials and downloadable bonuses100% satisfaction guarantee Your Singing Instructor: Faustine Parsons Communication expert Faustine is a manager, speaker and communication expert. She has over 14 years of experience in coaching and developing people skills for SMEs and international corporations. As a manager, she saw firsthand the importance of an effective team cooperation and got involved in the field. As a consultant, she specialized in smoothing the information exchange, bringing down barriers and creating a positive atmosphere throughout the organization. She developed skills and techniques which solved many of her clients’ problems. She combines the theory of psychology with down to earth practical approach. Let her guide you during the exciting journey through the dynamics of human interactions. Get ready for an interesting and fun ride, as her passion and positivity is contagious from the very beginning. Overview of the course: Detailed video presentations and training videos Fundamentals of effective communication Emotions and problems Daily communication at work and at home Business environment Advanced subjects Repetitions and tests Five repetitions Five tests Final exam Exercises and practial tasks Mapping good and bad practices Recognizing styles, behaviors and personalities Troubleshooting and dispute resolving in practice Giving feedback Overcoming challenges in your position All in one simulation Bonus materials Best practices kaizen map Scenarios and phrases for everyday situations Communication no-nos guide Personalities compendium Experience Beginner-IntermediateLanguage English, German, French, Polish, Portuguese A letter from your expert instructor: Many professionals encounter communication problems both at home and at work. They manifest themselves in misunderstandings, disputes and tension. It does not have to be this way. It all boils down to communication skills. For people and organizations who master them, everything comes easy.In my career, I have seen tremendous transformations on this front. Join me in this unique course and let me show you many of the best practices and techniques to make your life easier. Believe in yourself and make the most of your opportunities. Invest in your communication skills today!Faustine ParsonsCommunication expert

Business Administration Course
By KEMP CENTER
Master the secrets of effective business administration and achieve success! Do you want to be a good Administrator? Learn new and improve your business administration skills Upskill yourself; improve your business administration skills and advance your career Learn from the experts The course is delivered by an expert trainer of business administration with many years of experience in the field Test your knowledge Successfully complete the practical tasks and tests through the course and the final exam to consolidate your knowledge and skills Do you want to improve your knowledge of business administration and gain new practical skills? Do you like to change your career and move into a business administration role? Are you looking to upskill and get a valuable certificate that you can add to your CV? We are pleased to inform you that we have answer to all your requirements, offering you professionally designed, advanced business administration course that will make you proficient in the most important aspects of business administration so you can enjoy limitless career prospects worldwide. This is a great opportunity to study at any time in the comfort of your own home and at your own pace from any device that has internet connection. It is ideal for those with insufficient time to commit to traditional training or career development. It is designed for people looking to begin a career in business administration and also for those that want further improve their existing careers in the field. The course begins with an introduction to modern business administration; and then explores the fundamentals of administration management, the business environment; professional HR management; management of business resources and the performance management. You will also learn about the importance of business communication in a business environment and how to develop an effective corporate communication; how to write professional report and how to build and maintain effective customer relations. Developing and managing budget, creating effective marketing plan will be easy tasks for you after completing the course. The course will be delivered through a multimedia platform Kemp Center and will eventually bring you valuable certificate of completion that you can add to your CV. Invest today in yourself so you won’t regret tomorrow! Join us now! Join the Business Administration course and get the benefits: Learning from a professional instructor24/7 access from any devicePractical exercisesTests, quizzes and recapsCertificate of completionExtra materials and downloadable bonuses100% satisfaction guarantee Your Singing Instructor: Malaika Masozi Business Administration Expert The Business administration course will be delivered by an expert in business administration, the best one in the field with many years experience and active role in business administration. The expert trainer will share all the knowledge and experience with you, will introduce you the business administration completely and further teach you about the HR, performance, finance, resources and risk management. You will learn how to conduct effective marketing, how to build an effective corporate communication and customer relation and how to write professional report in practice. You will gain knowledge that is required to ensure you can successfully work in different environments in a business administration role. Your expert trainer will assist you and guide you through several knowledge repetitions, practical tasks and tests to make sure you remember all the important information and to consolidate your gained knowledge even better. At the end, you will be capable to use everything you learnt in practice while starting a new business. The Business administration course includes: I. Video presentations Practical knowledge about business administration Business administrations, business environment, HR Management, performance and team management, finance and risk management, business communication and building and maintaining customer relations etc. Real examples of business environments Professional HR planning; implementing different techniques for employees’ managing; managing finances and resources; conducting effective marketing; professional report writing; developing working relationships with colleagues; building effective customer relations; identifying risks and implementing risk strategies; starting a business. II. Practical part Training videos and video tutorials In the course, you will find many training videos and video tutorials that will teach you and will give you practical advices how to implement successfully the gained knowledge from the course in practice III. Knowledge repetitions and tests Repetitions of acquired knowledge and tests Through the course, you will have 10 repetitions and 10 tests that will allow you to check the acquired knowledge and better remember the topics discussed in the course. Final test Passing the final test means that you have enough knowledge and skills in the field of business administration, and that you became certified business administration expert. IV. Tasks Practical tasks about: Making changes in the business environment HR planning Implementing techniques for employees’ better-managing Developing and managing budget Implementing workable resource management strategy Creating effective marketing plan Professional report writing Building effective team Transforming negative customer experience into positive one Identifying risks and implementing risk strategies V. Bonus materials for the course Additional materials for use in daily work You will receive from us, among other things, a practical guide how to perform business administration tasks successfully and effectively and how to improve your skills. Experience Beginner-IntermediateLanguage English, German, French, Polish, Portuguese A letter from your expert instructor: From the largest international corporations to the smallest local offices, strong administration plays a crucially important role in every business and organization. The quality and competency of the administrative duties reflect the successfulness of the business, so, those who perform the business administration tasks should perform their duties on the highest professional level.We are pleased to present you and invite you to most popular and advanced study program for business administration, a premium course that will help you develop essential knowledge of how to perform administrative tasks and how to apply your knowledge in a variety of industries and job roles.Learn from the best in the business administration field, from our expert trainer who will share his/her knowledge and experience with you, so you can benefit from it.The course will help you to learn effective working practices that are applicable to office and administration roles, will improve your practical skills and performance in the workplace, will help you to develop and further improve your knowledge at a range of a master of business administration expert.The course package contains extensive material presented in an interesting form, learning by using specific examples, knowledge repetitions, varied practical tasks, final exam, and on top of everything, getting valuable certificate of completion at the end of the course. And all this is available to all people anywhere in the world with internet access, delivered on the Kemp Center platform.Study flexible – when you like and at your own pace – for a qualification that could transform your career prospects for life.Join us now!Malaika MasoziBusiness Administration Expert
