- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Courses delivered Online
We couldn't find any listings for your search. Explore our online options and related educators below to see if they help you.
Know someone teaching this? Help them become an Educator on Cademy.
Online Options
Show all 4491Remote Policy Evaluation Methods February 2025
By Institute for Fiscal Studies
The course covers research design principles and all main quantitative evaluation methods: randomised experiments, instrumental variables, sharp and fuzzy regression discontinuity designs, regression methods, matching methods and longitudinal methods (before-after, difference-in-differences and synthetic controls).

META-Health Professional 2025
By META-Health International
Become a META-Health professional with our 6 months intensive training! Do you want to learn the scientific background of body-mind-interactions and how to integrate this knowledge in your practical work with your clients? This course contains both self-study and live training in online classes and covers the META-Health Level 1 and 2 material. It will take 6 months including 24 live sessions, 2 hours each, mainly in a weekly rhythm. You will get preparatory videos, reading material and tasks that help to understand and integrate the content, while the group sessions focus on demonstrations, discusion of the material, and practical exercises for you. All the time, our trainers and tutors will support you and we’ll be a learning family with an active chatgroup. Altogether you should reckon approximately 5 hours per week.

Zen Yoga, Thursdays 10am (ONLINE)
By Mark Westmoquette
Join Mark on Thursday mornings to move, explore, and wake up to your true nature. Mark's classes are all about re-connecting the body and mind in order to find a sense of health, integration, freedom and kindness. Most of the time his classes are based around the seasons, since the Chinese energy system is very connected to the time of year and changing seasons.

Pilates - Mixed Ability - ONLINE
By For A Better You - Pilates & Pelvic Floor Health
Pilates is a great way to get your body moving, with low resistance body weight exercises it is suitable for any fitness level. If you work from home or are just wanted to improve yourself then look no further

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Selling Skills ► Into Action strengthens participants’ ability to apply a consultative, client-focused approach to the selling process. It is exercise-based, highly interactive and designed to yield results that can be put to use immediately. Outcomes – Participants will be able to: Identify and reach the most promising prospects to increase efficiency, Research and understand each unique customer to demonstrate expertise and ability, Conduct productive sales meetings that yield the best data to present the most effective solution, Present solutions that are fully aligned with the prospect’s situation and needs, and Work more effectively and resourcefully to close more business. ONLINE—Selling Skills ► Into Action is a 4-hour interactive online course. Register for this class and you will be sent ONLINE login instructions prior to the class date. Dr. Atkins, thank you for sharing ‘Language of Happiness and Power of Praise‘ with our chapter! We enjoyed the interactive presentation and your professionalism. I received positive feedback from our members—there is nothing better than that! Thanks again. Olga Otero, Chapter PresidentHuman Resources Association of Palm Beach County (HRPBC)

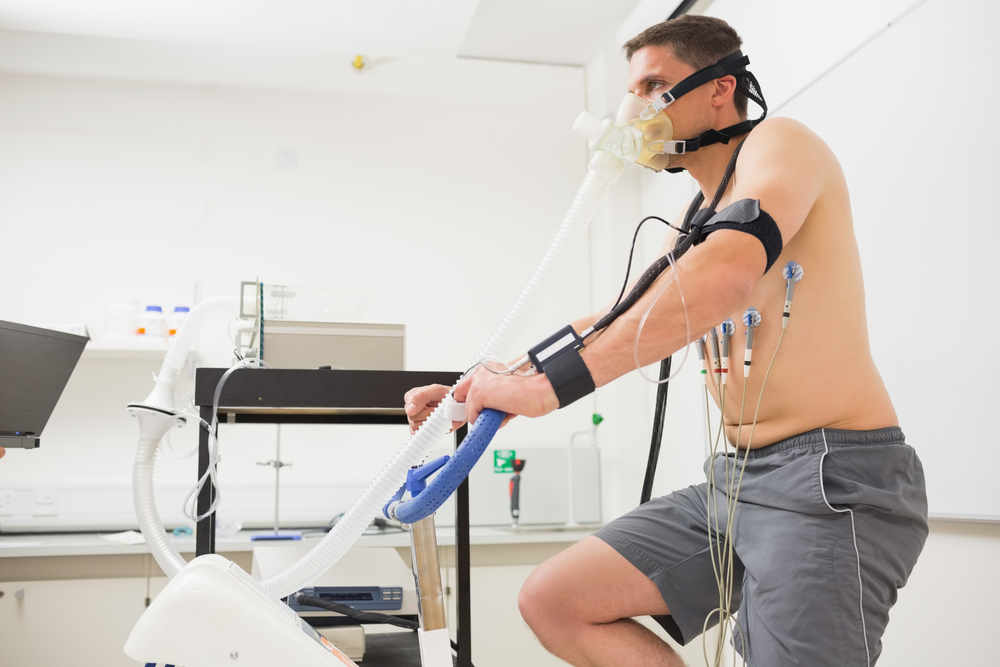
Discover the intricate mechanisms behind human movement and physical performance in our comprehensive online course. Explore anatomy, bioenergetics, cardiovascular health, and training principles. Perfect for aspiring coaches, trainers, and sports science enthusiasts. Enroll now and unlock the secrets to optimizing athletic potential!
Enrolment for the Women's Voice Medicine Journey & 3 x 1-1 Coaching Sessions
5.0(39)By Sing for Your Soul
Welcome to the Women's Voice Medicine Journey. This is a female-designed, step-by-step programe, to teach you how you can truly activate and integrate some of the most essential Embodied Voicework tools to Free Your Voice and unleash your Creative Feminine Power.
Welcome to the Women's Voice Medicine Journey. This is a female-designed, step-by-step programe, to teach you how you can truly activate and integrate some of the most essential Embodied Voicework tools to Free Your Voice and unleash your Creative Feminine Power.

