- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
143 Courses delivered Online
An Introduction to Emotional Intelligence at Work
By Workplace Innovation Europe
This CPD accredited short course is for everyone who wants to work more effectively with their collaeagues. It will help strengthen personal competencies including listening and communication, understanding and engaging with different perspectives, and working together to create a positive culture in the office or on the shopfloor.

The 18th edition course is an online short course aimed at anyone involved in the design, construction and inspection and testing of electrical systems, also a nationally recognised ‘must have’ qualification for companies seeking to recruit electricians. It is intended to introduce the candidate to a working knowledge of the Wiring Regulations along with a brief overview of the Electricity at Work Regulations covering their statutory duties. Previous knowledge of the regulations is not assumed, however, a fundamental knowledge of electrical principles is assumed.

The Central Heating Controls Wiring and Fault Finding course is a two day-day short course aimed at anyone involved in the construction, commissioning, inspection & testing or maintenance of central heating electrical control systems. The Central Heating Controls Wiring and Fault Finding course covers all the commonly used control systems in use today and is focused on the ‘practical’ construction and commissioning along with the relevant fault finding techniques. The Central Heating Controls Wiring and Fault Finding course requires an understanding of electrical principles and cable termination skills. A requirement of this course is the successful completion of their Essential Electrics examination prior to sitting the central heating controls wiring & fault finding examinations. Please ring if clarification is needed on this point. There are two 20 minute open book examinations and the associated practical assessments. A third examination and associated assessment will be required for candidates not holding the Essential Electrics unit. The Central Heating Controls Wiring and Fault Finding course comprises of: Short theory sessions introducing the conventional wiring systems, ‘Y’ Plan, ‘S’ Plan, ‘C’ Plan, Etc. Detailed practical workshop sessions, undertaking the construction, testing and commissioning of systems Fault finding and maintenance tasks performed on working systems Examination and practical assessment preparation ready for the assessments Evaluation of the system control function (why and how it works) Each student will work on their own system, and will have plenty of time to absorb and understand how each systemworks. Advise will be given on suitable ‘tooling’ and test equipment. These are nationally recognizable qualifications which are fast becoming an essential requirement for this type of work. The course costs include comprehensive course notes and examination entry fees.

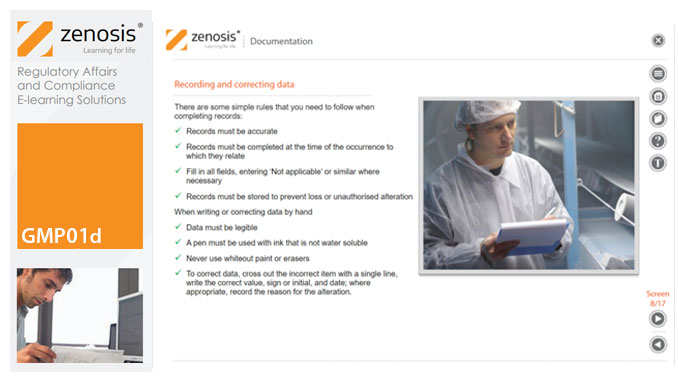
GMP01d - Documentation
By Zenosis
Comprehensive documentation of procedures, formulas, work instructions, and specifications, and thorough recording of batch data, are fundamental requirements of GMP. In this short course we explain why documentation is so important, identify different types of document required, and set out some simple rules for recording and correcting data.
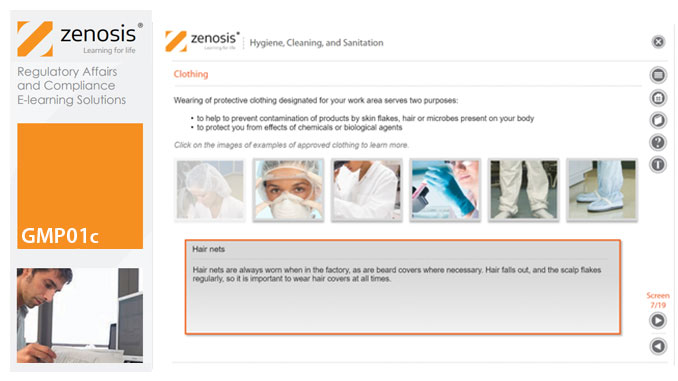
GMP01c - Hygiene, cleaning, and sanitation
By Zenosis
Prevention of contamination is one of the most important goals of GMP. Contamination of product is often difficult to detect, so GMP rules emphasise preventive measures, including: attention to personal health and hygiene, and the wearing of special clothing, by staff; and cleaning and sanitation of premises and equipment. In this short course we set out the basics of GMP requirements in these vital areas.
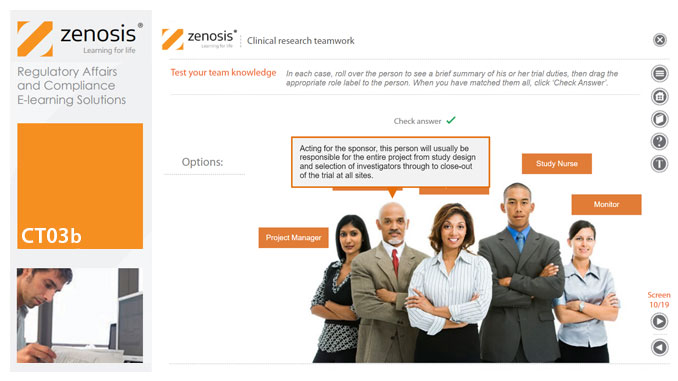
CT03b - Clinical research teamwork
By Zenosis
A clinical trial, particularly a late-phase commercial study, is a major project requiring collaboration between the sponsor and staff or contractor, on the one hand, and the clinical investigator(s) and other healthcare professionals on the other. Good communication among all parties is essential. In this short course we introduce the major roles in a typical clinical research project and outline their duties.
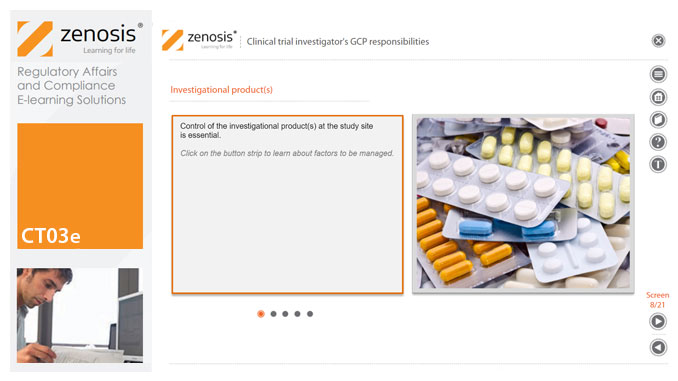
CT03e - Clinical trial investigator’s GCP responsibilities
By Zenosis
A clinical investigator is responsible for conducting the clinical trial in compliance with the study protocol, GCP, medical ethics, and applicable legal requirements. The clinical research community expects that investigators and clinical staff are fully trained in GCP. Duties and functions discussed in this short course include: provision of adequate resources; liaison with IRB/IEC; compliance with protocol; management of investigational product(s), informed consent and data records; and safety reporting.
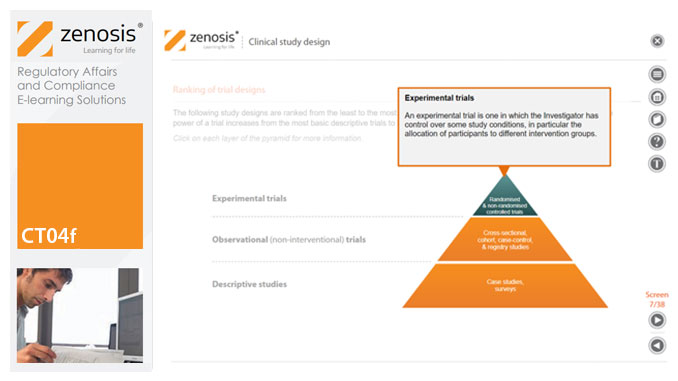
CT04f - Clinical study design
By Zenosis
Clinical trial design establishes the framework upon which the clinical trial process will be conducted, and sets the objectives of the trial. The application for marketing approval, submitted to the regulatory authorities, will provide clinical data reflecting the trial design. Since trial design impacts the whole drug development process and lifecycle, particular care and due diligence is essential. This short course provides an overview of the main types of study design.
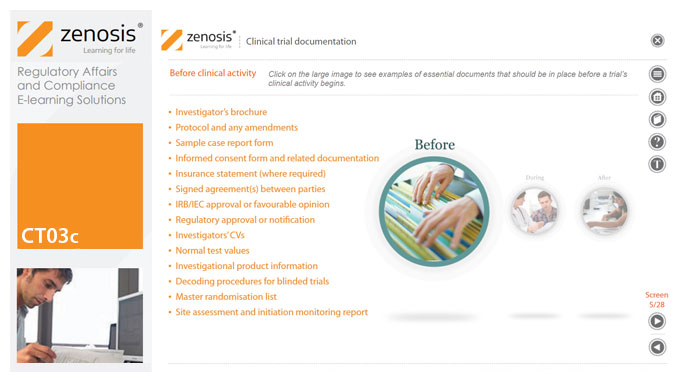
CT03c - Clinical trial documentation
By Zenosis
Regulatory authorities tend to abide by the maxim that ‘If it isn’t documented, it didn’t happen’. Rigorous documentation of all aspects of a clinical trial is necessary to provide evidence of GCP and compliance with regulatory requirements, as well as enabling effective management of the trial. In this short course we describe important examples of the documents designated by ICH GCP as essential to the conduct of a clinical trial.
Preparing for a Job Interview-CPD Approved
By BAB Business Group
Interviews are two-way processes, where the employer and the interviewee can both decide if they are a good fit. This short course will provide you with some useful tips for how to prepare for an interview including preparing answers to common questions. The course also covers other things to consider in advance of the interview including planning how you will get there. It briefly covers some of the key differences between online and face to face interviews.
