- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7570 Courses delivered Online
VTCT Level 3 Award in Anatomical and Physiological Knowledge of Body Systems Online Course
By Cosmetic College
Designed to provide you with an in-depth understanding of the main systems of the body, this course covers a range of topics including skin, nails, and hair, the skeletal system, the muscular system, the nervous system, the endocrine system, the respiratory system, the cardiovascular system, the lymphatic system, and the digestive system. At a price of just £499, this online course is an affordable way to gain valuable knowledge and understanding of the human body, which is essential in many beauty and complementary therapy qualifications. Whether you have previous experience or not, this course is suitable for everyone. Our expert tutors will guide you through each module, providing you with the knowledge and skills you need to succeed. Upon completion of this course, you will not only have gained a solid understanding of the human body, but also have the opportunity to explore further career pathways in allied healthcare fields. Don't miss out on this fantastic opportunity to enhance your knowledge and take the first step towards your dream career. Sign up now and start your learning journey with us! Additional course details Course Features VTCT QualificationVetted accredited trainingFully Online TrainingTrain your way on any deviceFull DemonstrationComplete end to end treatment demonstrationQualificationSchedule online exam after completion
Level 3 Diploma in Health and Social Care
By Kingston Open College
Level 3 Diploma in Health and Social Care | 5 Additional CPD accredited courses | No Hidden FEE

***24 Hour Limited Time Flash Sale*** Pharmacy Skills, Medicine Management & Medical Law Admission Gifts FREE PDF & Hard Copy Certificate| PDF Transcripts| FREE Student ID| Assessment| Lifetime Access| Enrolment Letter Begin your journey towards a rewarding and successful career by enrolling in our all-inclusive bundle of 8 Pharmacy Skills, Medicine Management & Medical Law courses. At UKHF Online, we have carefully selected and combined these courses to equip you with the vital skills and knowledge necessary to thrive in Pharmacy Skills, Medicine Management & Medical Law. Whether you're a student, recent graduate, or job seeker, our Pharmacy Skills, Medicine Management & Medical Law bundle is designed to enhance your CV, impress potential employers, and set you apart from the competition. Key Features of the Pharmacy Skills, Medicine Management & Medical Law Bundle: 3 QLS-Endorsed Courses: We proudly offer 3 QLS-endorsed courses within our Pharmacy Skills, Medicine Management & Medical Law bundle, providing you with industry-recognized qualifications. Plus, you'll receive a free hardcopy certificate for each of these courses. QLS Course 01: Pharmacy Skills QLS Course 02: Medical Law QLS Course 03: Safe Handling of Medicines 5 CPD QS Accredited Courses: Additionally, our bundle includes 5 relevant CPD QS accredited courses, ensuring that you stay up-to-date with the latest industry standards and practices. Course 01: Phlebotomist Training Course 02: Medical & Clinical Administration Diploma Course 03: Clinical Observation Skills for Carers Course 04: Nurse Prescribing Diploma Course 05: Emergency Medicine - Paramedicine In Addition, you'll get Five Career Boosting Courses absolutely FREE with this Bundle. Course 01: Professional CV Writing Course 02: Job Search Skills Course 03: Self Esteem & Confidence Building Course 04: Professional Diploma in Stress Management Course 05: Complete Communication Skills Master Class Convenient Online Learning: Our Pharmacy Skills, Medicine Management & Medical Law courses are accessible online, allowing you to learn at your own pace and from the comfort of your own home. Learning Outcomes of the Pharmacy Skills, Medicine Management & Medical Law Bundle: Master the foundational principles and techniques of Pharmacy Skills, Medicine Management & Medical Law. Develop advanced proficiency in Pharmacy Skills, Medicine Management & Medical Law methodologies and strategies. Acquire in-depth knowledge of the latest trends and advancements in Pharmacy Skills, Medicine Management & Medical Law. Enhance your problem-solving and critical thinking abilities within the context of Pharmacy Skills, Medicine Management & Medical Law. Cultivate strong communication and collaboration skills essential for success in Pharmacy Skills, Medicine Management & Medical Law. The Pharmacy Skills, Medicine Management & Medical Law bundle is a comprehensive collection of courses that have been meticulously designed to provide you with a well-rounded education in Pharmacy Skills, Medicine Management & Medical Law. With a combination of 3 QLS-endorsed courses and 5 CPD QS-accredited courses, this bundle offers you the perfect balance of essential knowledge and valuable skills. What's more, we are proud to offer free hardcopy certificates for each course within the Pharmacy Skills, Medicine Management & Medical Law bundle, giving you the recognition you deserve. CPD 250 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This bundle is ideal for: Individuals interested in pursuing a career in the pharmaceutical industry Professionals in the healthcare industry looking to enhance their knowledge and skills Individuals who want to expand their knowledge and stay updated on industry trends Anyone interested in learning about pharmacy skills, medicine management, and medical law. Career path This bundle will be beneficial to anybody planning to start a career as: Pharmacy Technician: £20,000 - £28,000 Clinical Pharmacist: £36,000 - £80,000 Nurse Prescriber: £29,000 - £53,000 Medical Administrator: £18,000 - £30,000 Phlebotomist: £17,000 - £23,000 Emergency Medical Technician: £18,000 - £31,000 Certificates Digital certificate Digital certificate - Included Hard copy certificate Hard copy certificate - Included

***24 Hour Limited Time Flash Sale*** Dermatology, Skincare with Nutrition and Hydration Admission Gifts FREE PDF & Hard Copy Certificate| PDF Transcripts| FREE Student ID| Assessment| Lifetime Access| Enrolment Letter Begin your journey towards a rewarding and successful career by enrolling in our all-inclusive bundle of 8 Dermatology, Skincare with Nutrition and Hydration courses. At UKHF Online, we have carefully selected and combined these courses to equip you with the vital skills and knowledge necessary to thrive in Dermatology, Skincare with Nutrition and Hydration. Whether you're a student, recent graduate, or job seeker, our Dermatology, Skincare with Nutrition and Hydration bundle is designed to enhance your CV, impress potential employers, and set you apart from the competition. Key Features of the Dermatology, Skincare with Nutrition and Hydration Bundle: 3 QLS-Endorsed Courses: We proudly offer 3 QLS-endorsed courses within our Dermatology, Skincare with Nutrition and Hydration bundle, providing you with industry-recognized qualifications. Plus, you'll receive a free hardcopy certificate for each of these courses. QLS Course 01: Dermatology QLS Course 02: Nutrition and Hydration QLS Course 03: Skincare 5 CPD QS Accredited Courses: Additionally, our bundle includes 5 relevant CPD QS accredited courses, ensuring that you stay up-to-date with the latest industry standards and practices. Course 01: Gel Manicure Course 02: Luxury Spa Facial Therapy Course - Step-by-Step Guide Course 03: Fashion Store Assistant Training Course 04: Personal Hygiene Course 05: Indian Bridal Makeup In Addition, you'll get Five Career Boosting Courses absolutely FREE with this Bundle. Course 01: Professional CV Writing Course 02: Job Search Skills Course 03: Self-Esteem & Confidence Building Course 04: Professional Diploma in Stress Management Course 05: Complete Communication Skills Master Class Convenient Online Learning: Our Dermatology, Skincare with Nutrition and Hydration courses are accessible online, allowing you to learn at your own pace and from the comfort of your own home. Learning Outcomes of the Dermatology, Skincare with Nutrition and Hydration Bundle: Master the foundational principles and techniques of Dermatology, Skincare with Nutrition and Hydration. Develop advanced proficiency in Dermatology, Skincare with Nutrition and Hydration methodologies and strategies. Acquire in-depth knowledge of the latest trends and advancements in Dermatology, Skincare with Nutrition and Hydration. Enhance your problem-solving and critical thinking abilities within the context of Dermatology, Skincare with Nutrition and Hydration. Cultivate strong communication and collaboration skills essential for success in Dermatology, Skincare with Nutrition and Hydration. The Dermatology, Skincare with Nutrition and Hydration bundle is a comprehensive collection of courses that have been meticulously designed to provide you with a well-rounded education in Dermatology, Skincare with Nutrition and Hydration. With a combination of 3 QLS-endorsed courses and 5 CPD QS-accredited courses, this bundle offers you the perfect balance of essential knowledge and valuable skills. What's more, we are proud to offer free hardcopy certificates for each course within the Dermatology, Skincare with Nutrition and Hydration bundle, giving you the recognition you deserve. CPD 260 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Beauty and skincare enthusiasts Those looking to start a career in the beauty industry Those looking to expand their knowledge in skincare and dermatology Healthcare professionals looking to broaden their knowledge Anyone interested in learning about the relationship between diet and skin health Career path Beauty Therapist - £20,000 - £30,000 Dermatologist - £40,000 - £70,000 Skincare Specialist - £20,000 - £35,000 Aesthetician - £20,000 - £30,000 Spa Manager - £25,000 - £40,000 Certificates Digital certificate Digital certificate - Included Hard copy certificate Hard copy certificate - Included

Tired of browsing and searching for a Schizophrenia Awareness course you are looking for? Can't find the complete package that fulfils all your needs? Then don't worry as you have just found the solution. Take a minute and look through this extensive bundle that has everything you need to succeed. After surveying thousands of learners just like you and considering their valuable feedback, this all-in-one Schizophrenia Awareness bundle has been designed by industry experts. We prioritised what learners were looking for in a complete package and developed this in-demand Schizophrenia Awareness course that will enhance your skills and prepare you for the competitive job market. Also, our experts are available for answering your queries on Schizophrenia Awareness and help you along your learning journey. Advanced audio-visual learning modules of these Schizophrenia Awareness courses are broken down into little chunks so that you can learn at your own pace without being overwhelmed by too much material at once. Furthermore, to help you showcase your expertise in Schizophrenia Awareness, we have prepared a special gift of 1 hardcopy certificate and 1 PDF certificate for the title course completely free of cost. These certificates will enhance your credibility and encourage possible employers to pick you over the rest. This Schizophrenia Awareness Bundle Consists of the following Premium courses: Course 01: Schizophrenia Awareness Course 02: Depression Counselling Training Course 03: Mental Health Support Worker Course 04: Anxiety Management Course 05: Mental Health Nursing Diploma Course 06: Mental Health Laws and Awareness - Level 2 Course 07: Medication Administration Level 4 Course 08: Palliative and End of Life Care Training- Level 5 Course 09: Cognitive Behavioural Therapy (CBT) Training Course 10: Addiction and Mental Health - Dual Diagnosis Course 11: Dialectal Behaviour Therapy (DBT) Course 12: Release Addiction, Anxiety and Trauma Level-3 Course 13: Food and Mood: Improving Mental Health Through Diet and Nutrition Course 14: Mental Health First Aid Certification Enrol now in Schizophrenia Awareness to advance your career, and use the premium study materials from Apex Learning. The bundle incorporates basic to advanced level skills to shed some light on your way and boost your career. Hence, you can strengthen your Schizophrenia Awareness expertise and essential knowledge, which will assist you in reaching your goal. Course Curriculum: (Title Course Only) Schizophrenia Awareness: Module 01: Introduction to Schizophrenia Module 02: Symptoms of Schizophrenia Module 03: Causes of Schizophrenia Module 04: Diagnosis of Schizophrenia Disorder Module 05: Treatment of Schizophrenia Module 06: The Rehabilitation of Schizophrenia Module 07: How Family Members Should Deal with Schizophrenia Module 08: How to Create a Supportive Environment Module 09: Improving the Quality of Life CPD 140 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone from any background can enrol in this Schizophrenia Awareness bundle. Requirements Our Schizophrenia Awareness course is fully compatible with PCs, Macs, laptops, tablets and Smartphone devices. Career path Having this Schizophrenia Awareness expertise will increase the value of your CV and open you up to multiple job sectors. Certificates Certificate of completion Digital certificate - Included You will get the PDF Certificate for the title course Schizophrenia Awareness absolutely Free! Other PDF Certificates are available for £6 each. Certificate of completion Hard copy certificate - Included You will get the Hard Copy certificate for the title course Schizophrenia Awareness absolutely Free! Other Hard Copy certificates are available for £10 each. Please Note: The delivery charge inside the UK is £3.99, and the international students must pay a £9.99 shipping cost.

VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL03: Commissioning and Installation Qualification
By Zenosis
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
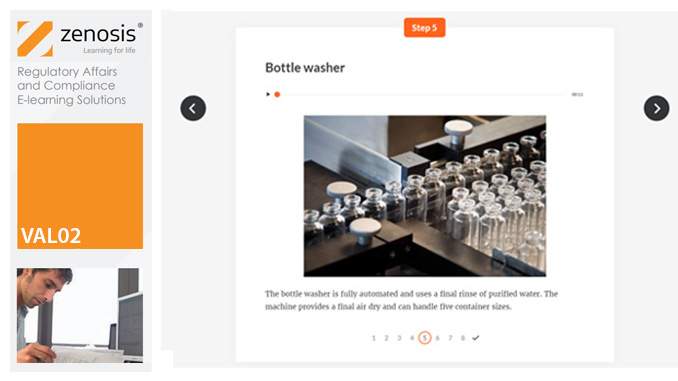
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
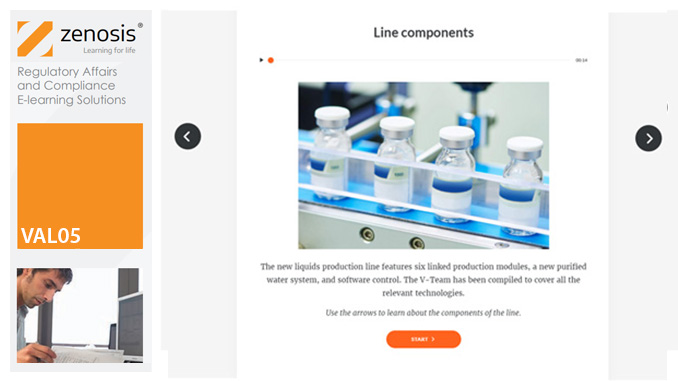
VAL05: Equipment Cleaning Validation
By Zenosis
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.
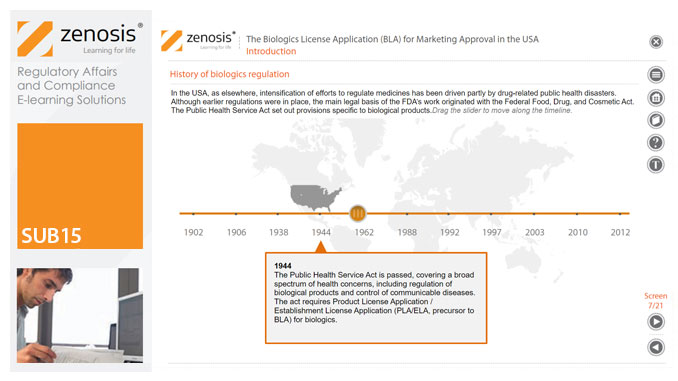
SUB15: The Biologics License Application (BLA) for Marketing Approval in the USA
By Zenosis
This module describes the requirements that must be met to obtain licensure of a biological product. Subjects covered include the regulatory context, the content and format of the BLA submission, the review process, and provisions for expedited development and review.