- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
5651 Courses delivered Online
Carbon Capture, Utilization & Storage (CCUS)
By EnergyEdge - Training for a Sustainable Energy Future
About this Virtual Instructor Led Training (VILT) This 5 half-day Virtual Instructor Led Training (VILT) course covers carbon capture and geological storage of carbon dioxide. Burning fossil fuels for energy is a major source of carbon dioxide emissions to the atmosphere. Most anthropogenic (man-made) carbon dioxide is emitted by coal-fired or gas-fired power plants, and significant quantities of carbon dioxide are emitted through the production and separation of carbon dioxide-rich natural gas and industries such as cement, iron and steel. Carbon Capture Utilization and Storage, or CCUS, involves the long-term storage of captured carbon dioxide emissions in subsurface geologic formations. This VILT course covers all aspects of CCUS including transport, storage and monitoring, economics and community engagement. It explores in detail the challenges of the current technology of geological storage, monitoring and verification including examples from working projects around the world. Many of these technologies are commonly employed by the petroleum industry. Successful deployment of CCUS will also require economic incentives, appropriate regulation, clarity on liability issues and acceptance by the community. These aspects of CCUS, and the corresponding opportunities for appropriately skilled organisations and individuals also will be discussed. Course Content at a Glance Context for CCS/CCUS as An Emissions-reduction Measure Principles of Geological Storage Finding Geological Storage Sites Stationary Sources of Carbon Dioxide for Capture Carbon Dioxide Capture Technologies Compression and Transport of Carbon Dioxide Economics of CCS/CCUS Community, Safety, Legal & Regulatory Issues Risk Assessment Training Objectives Upon completion of this VILT course, participants will be able to: Identify the need for Carbon Capture and Storage (CCS) Outline the key steps in the Carbon Capture and Storage process Distinguish between reservoir rocks and sealing rocks Describe the importance of permeability and porosity to storing carbon dioxide Contrast the geological structures and trapping mechanisms for storing carbon dioxide Describe the changes in geologically stored carbon dioxide over time Outline the monitoring techniques employed to ensure the carbon dioxide is safely stored Appreciate the industrial applications of carbon dioxide capture Recognize the scale of industry required for transporting and storing carbon dioxide Describe economic considerations for CCS/CCUS Outline the economic and environmental opportunities and challenges with using carbon dioxide injection in a range of applications Explain the challenges of regulatory frameworks and public acceptance in a CCS/CCUS project Identify potential risks of a CCS/CCUS project Outline the risk assessment and management process Target Audience This VILT course is ideally suited for a technical audience - geoscientists, petroleum and chemical engineers - as well as for economists, regulators, legal staff and managers wishing to learn more about the details of both the technical, regulatory and socio-economic aspects of carbon capture and storage. Participants should have: Experience with oil and gas, coal or other energy projects Basic understanding of the energy industry Course Level Intermediate Trainer Your first expert course leader spent 18 years in the Petroleum Industry before joining academia, in both technical and managerial roles with Shell, Arco and Vico. He has received numerous awards, including Distinguished Service, Honorary member and Special Commendation awards from the American Association of Petroleum Geologist (AAPG) and was AAPG's International Vice-President and recently chairman of AAPG's House of Delegates (the Associations Parliamentary body). He is an SPE Distinguished Lecturer (DL) and has served as DL for several other professional organisations, including, AAPG, IPA and PESA. He is currently a Professor of Petroleum Geology and Engineering at the Australian School of Petroleum, University of Adelaide. He holds the South Australia State Chair in Carbon Capture & Storage (CCS) and is also presently Distinguished Scientist of the Cooperative Research Centre for Greenhouse Gas Technologies (CO2CRC), having served earlier as the Storage Program Manager and Chief Scientist. Your second expert course leader has a wide and deep knowledge of major capture technologies: solvent, membrane and adsorption based technologies and has developed pathways for retrofitting CO2 capture and storage (CCS) to fossil fuel-based power plants. He has been actively engaged in Post-combustion capture project management and demonstration projects in Victoria's Latrobe Valley on CO2 capture and hydrogen production, and on CO2 capture using membrane contactor technology. He has led various feasibility studies for the Asian Development Bank on CO2 Capture at Indian Oil Corporation's refineries, for JPOWER on hydrogen production from Victorian brown coal and for Kawasaki on incorporation of CCS in hydrogen production from fossil fuel. He has authored multiple peer reviewed journal articles, co-authored various confidential reports on CO2 capture, utilization and hydrogen production and utility, and has presented his work at various conferences, symposiums and seminars. He has a PhD in Chemical Engineering from Monash University Australia and a Master of Technology in Process Engineering from Indian Institute of Technology Delhi India. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information about post training coaching support and fees applicable for this. Accreditions And Affliations

Introducing GDPR-CPD Approved
By BAB Business Group
The General Data Protection Regulation (GDPR) is designed to strengthen and unify the principles of data protection for all individuals within the European Union and the European Economic Area. The GDPR is an incredibly important change to data privacy regulations so understanding its correct implementation is vital for all UK businesses and organisations, and particularly for staff who regularly deal with personal data. This online course is designed specially for those front line staff and provides a clear introduction to the main elements of the GDPR, including compliance and the consequences of non-compliance. It explains the roles of key players - Data Protection Officers, Data Controllers, Data Protection Leads and Data Processors and covers the main categories of personal data, along with the six lawful bases for processing data, and how to audit the data your organisation holds. Other topics examined include the Seven Principles of the GDPR and the Eight Rights for Individuals, along with the importance of your Privacy Policy - how to construct one, and how to use it effectively when dealing with data subjects. Finally, there's important information on data breaches; how to avoid them, what to do if one is discovered and how to file a breach report

48-Hour Knowledge Knockdown! Prices Reduced Like Never Before! This Diploma in Quantity Surveyor at QLS Level 4 course is endorsed by The Quality Licence Scheme and accredited by CPDQS (with 120 CPD points) to make your skill development & career progression more accessible than ever! Statistical data shows that the construction industry is growing rapidly, with a projected 2.7% growth rate in 2023 in the UK alone. As the industry expands, the demand for skilled Quantity Surveyors increases. Our Quantity Surveyor course offers an in-depth curriculum covering all aspects of the role, equipping you with the knowledge and skills necessary to succeed in this dynamic industry. The Quantity Surveyor course covers modules on measurement, cost management, contract management, procurement, risk management, and more. You will learn how to predict potential risks, control costs, and write comprehensive reports. Upon completion of this Quantity Surveyor course, you will be well-versed in the role of a Quantity Surveyor, and ready to take on the challenges and opportunities in the industry. Learning Outcomes of Quantity Surveyor at QLS Level 4: Understand the history and development of Quantity Surveying Learn to measure and estimate costs accurately Understand procurement and contract management Analyze potential risks and manage them effectively Learn how to prepare bills and write comprehensive reports Gain knowledge of regulation and control within the industry Why Prefer This Quantity Surveyor at QLS Level 4 Course? Opportunity to earn a certificate endorsed by the Quality Licence Scheme & another accredited by CPDQS which is completely free. Get a free student ID card! (£10 postal charge will be applicable for international delivery) Innovative and engaging content. Free assessments 24/7 tutor support. Take a step toward a brighter future! *** Course Curriculum *** Here is the curriculum breakdown of the Quantity Surveyor at QLS Level 4 course: Module 01: Quantity Survey: An Introduction Module 02: Development of the Quantity Surveyor Module 03: Quantity Surveying Measurement Module 04: Cost Management Module 05: Pricing and Operational Estimation Module 06: Contract Management and Tendering Module 07: Procurement and Bill Preparation Module 08: Predicting Potential Risk and Management Module 09: Regulation and Control Module 10: Report Writing Assessment Process After completing an online module, you will be given immediate access to a specially designed MCQ test. The results will be immediately analysed, and the score will be shown for your review. The passing score for each test will be set at 60%. You will be entitled to claim a certificate endorsed by the Quality Licence Scheme after you have completed all of the Diploma in Quantity Surveyor at QLS Level 4 exams. CPD 120 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Quantity Surveyor course is for: Individuals interested in the construction industry Recent graduates seeking to enter the industry Professionals looking to upskill or change careers Those seeking to broaden their knowledge of Quantity Surveying Requirements No prior background or expertise is required. Career path The Quantity Surveyor at QLS Level 4 course will boost your CV and aims to help you get the job or even the long-awaited promotion of your dreams. Like as: Quantity Surveyor: £35,000 - £60,000 Senior Quantity Surveyor: £50,000 - £75,000 Commercial Manager: £55,000 - £85,000 Cost Manager: £35,000 - £65,000 Project Manager: £40,000 - £70,000 Estimator: £25,000 - £50,000 Certificates CPDQS Accredited Certificate Digital certificate - Included Diploma in Quantity Surveyor at QLS Level 4 Hard copy certificate - Included Show off Your New Skills with a Certificate of Completion After successfully completing the Diploma in Quantity Surveyor at QLS Level 4, you can order an original hardcopy certificate of achievement endorsed by the Quality Licence Scheme and also you can order CPDQSAccredited Certificate that is recognised all over the UK and also internationally. The certificates will be home-delivered, completely free of cost.

48-Hour Knowledge Knockdown! Prices Reduced Like Never Before! This Certificate in UK Pension System at QLS Level 3 course is endorsed by The Quality Licence Scheme and accredited by CPDQS (with 120 CPD points) to make your skill development & career progression more accessible than ever! Are you looking to improve your current abilities or make a career move? If yes, our unique UK Pension System at QLS Level 3 course might help you get there! It is an expertly designed course which ensures you learn everything about the topic thoroughly. Expand your expertise with high-quality training from the UK Pension System at QLS Level 3 course. Due to UK Pension System at QLS Level 3's massive demand in the competitive market, you can use our comprehensive course as a weapon to strengthen your knowledge and boost your career development. Learn UK Pension System at QLS Level 3 from industry professionals and quickly equip yourself with the specific knowledge and skills you need to excel in your chosen career. The UK Pension System at QLS Level 3 course is broken down into several in-depth modules to provide you with the most convenient and rich learning experience possible. Upon successful completion of the UK Pension System at QLS Level 3 course, an instant e-certificate will be exhibited in your profile that you can order as proof of your skills and knowledge. Add these amazing new skills to your resume and boost your employability by simply enrolling in this UK Pension System at QLS Level 3 course. This UK Pension System at QLS Level 3 training can help you to accomplish your ambitions and prepare you for a meaningful career. So, join us today and gear up for excellence! Why Prefer This UK Pension System at QLS Level 3 Course? Opportunity to earn a certificate endorsed by the Quality Licence Scheme & another accredited by CPDQS which is completely free. Get a free student ID card! (£10 postal charge will be applicable for international delivery) Innovative and engaging content. Free assessments 24/7 tutor support. Take a step toward a brighter future! *** Course Curriculum *** Here is the curriculum breakdown of the UK Pension System at QLS Level 3 course: Module 01: Overview of the UK Pension system Module 02: Type of Pension Schemes Module 03: Pension Regulation Module 04: Pension Fund Governance Module 05: Law and Regulation of Pensions in the UK Module 06: Key Challenges in UK Pension System Assessment Process You have to complete the assignment questions given at the end of the course and score a minimum of 60% to pass each exam. Our expert trainers will assess your assignment and give you feedback after you submit the assignment. You will be entitled to claim a certificate endorsed by the Quality Licence Scheme after you have completed all of the Certificate in UK Pension System at QLS Level 3 exams. CPD 120 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This UK Pension System at QLS Level 3 course is perfect for highly motivated people who want to improve their technical skills and prepare for the career they want! Requirements UK Pension System at QLS Level 3 No prior background or expertise is required. Career path The UK Pension System at QLS Level 3 course will boost your CV and aims to help you get the job or even the long-awaited promotion of your dreams. Certificates Certificate in UK Pension System at QLS Level 3 Hard copy certificate - Included Show off Your New Skills with a Certificate of Completion After successfully completing the Certificate in UK Pension System at QLS Level 3, you can order an original hardcopy certificate of achievement endorsed by the Quality Licence Scheme andalso you can order CPDQSAccredited Certificate that is recognised all over the UK and also internationally. The certificates will be home-delivered, completely free of cost. CPDQS Accredited Certificate Digital certificate - Included

Life can feel like a never-ending rollercoaster—one moment you're calm, the next you're spiralling. If your emotions seem to have a mind of their own, the DBT for Emotional Wellness Course is here to help. Built on Dialectical Behaviour Therapy (DBT) techniques, this course dives into proven methods for managing intense emotions, improving relationships, and learning to respond to life's ups and downs with more balance. It’s not about pretending things are fine—it’s about understanding yourself and handling emotions without letting them run the show. This course is perfect for anyone tired of overthinking at 2am or second-guessing every decision. You’ll explore topics like distress tolerance, emotional regulation, mindfulness, and interpersonal effectiveness—all from the comfort of your own space. Whether you're looking to improve your mental wellbeing or simply want to feel a bit more in control, the DBT for Emotional Wellness Course offers tools you can use in everyday situations without needing a psychology degree to understand them. Key Features CPD Accredited FREE PDF + Hardcopy certificate Fully online, interactive course Self-paced learning and laptop, tablet and smartphone-friendly 24/7 Learning Assistance Discounts on bulk purchases Course Curriculum Module 1: Introduction to DBT Module 2: Mindfulness Skills Training Module 3: Emotion Regulation Skills Module 4: Interpersonal Effectiveness Skills Module 5: Distress Tolerance Skills Module 6: Application of DBT in Specific Populations Module 7: Implementation of DBT in Clinical Settings Module 8: Ethical and Professional Considerations in DBT Practice Learning Outcomes: Understand the foundations of Dialectical Behavior Therapy principles. Apply mindfulness skills to enhance emotional awareness and self-regulation. Cultivate effective strategies for regulating and managing emotions. Develop interpersonal skills to navigate complex relationships with confidence. Acquire distress tolerance techniques for coping with challenging situations. Demonstrate ethical considerations essential for a successful DBT practice. Accreditation This course is CPD Quality Standards (CPD QS) accredited, providing you with up-to-date skills and knowledge and helping you to become more competent and effective in your chosen field. Certificate After completing this course, you will get a FREE Digital Certificate from Training Express. CPD 10 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Mental health professionals Therapists Counsellors Psychologists Social workers Healthcare professionals Students in psychology-related fields Individuals passionate about emotional well-being Career path Clinical Psychologist Counselling Psychologist Mental Health Therapist Social Worker Psychotherapist Psychiatric Nurse Certificates Digital certificate Digital certificate - Included Once you've successfully completed your course, you will immediately be sent a FREE digital certificate. Hard copy certificate Hard copy certificate - Included Also, you can have your FREE printed certificate delivered by post (shipping cost £3.99 in the UK). For all international addresses outside of the United Kingdom, the delivery fee for a hardcopy certificate will be only £10. Our certifications have no expiry dates, although we do recommend that you renew them every 12 months.

Diploma in Project and Quality Management - Level 7 is a 120 credits qualification. Project and quality management can be used in a variety of industries to bring projects in on time, on budget and to the right quality. Project management elements of this Diploma in Project and Quality Management - Level 7 course will focus on management techniques and applications to help you plan and implement activities to complete projects to your customers expectations. Quality management elements of the course will help you make sure that the outcome of the project is fit for your customer's purposes. Program Overview: Diploma in Project and Quality Management - Level 7 Key Highlights of Diploma in Project and Quality Management - Level 7 qualification are: Program Duration: 9 Months (Fast Track Mode Available) Program Credits: 120 Designed for working Professionals Format: Online No Written Exam. The Assessment is done via Submission of Assignment Tutor Assist available Dedicated Student Success Manager Timely Doubt Resolution Regular Networking Events with Industry Professionals Become eligible to gain direct entry into relevant Masters Degree programme. Alumni Status No Cost EMI Option Key facts about Level 7 Diploma in Project and Quality Management 100% Online: Study online with the UK's leading online course provider. Global programme: Study anytime, anywhere using your laptop, phone or a tablet. Study material: Comprehensive study material and e-library support available at no additional cost. Payment plans: Interest free monthly, quarterly and half yearly payment plans available for all courses. Why choose LSBR ? Transform your career: Enrol today and make a life changing decision, get necessary knowledge to transform your career. Move up in your career: With our fully accredited course, you can move up to the next level in your organisation with ease. Upgrade your skills: Add value to your current educational profile and achieve skill sets to compete in your job role. Cost effective: Typically a British qualification starts from GBP £3000 per annum, but with LSBR, you enjoy savings of up to 60% on your educational investment. Assessments: Assessment is done through written assignments and/ or dissertation project. Support: We offer live tutor support via online chat and email for all qualifications delivered by us. Career ProgressionLearners completing the Diploma in Project and Quality Management - Level 7 can progress to: A University partner to complete a dissertation to then receive a full master's degree, or Level 8 Diploma qualification, or Directly into employment in an associated profession University ExemptionsLearners can progress to a number of universities to complete a Master's Degree after completing their Diploma in Project and Quality Management - Level 7. This generally requires completion of a Dissertation only. The pathways are an indication of a learner's progress towards a university degree and are based on the university's review of Eduqual's learning programmes and outcomes. As our EduQual accredited qualifications are approved and regulated by Ofqual (Office of the Qualifications and Examinations Regulation)and Scottish Credit and Qualifications Framework (SCQF), learners are also eligible to progress to a Top-Up Degree, Master's programme, or MBA at many universities in UK and Overseas with advanced standing. Our qualifications are credit-rated for the Scottish Credit and Qualifications Framework (SCQF), a globally-recognised national qualifications framework aligned to other recognised frameworks such as the European Qualifications Framework (EQF) and the Ofqual Regulated Qualifications Framework (RQF).

CILT Level 3 Award in Warehousing
By The Business School (UK) Ltd
The CILT Level 3 Award in Warehousing is designed to provide Learners with the knowledge and understanding of warehousing principles and to deliver the skills required by an aspiring warehouse manager to operate effectively. The qualification covers all of the key areas and activities of warehousing operations.

GCSE English Language Course - AQA English Language Online
By Study Plex
Highlights of the Course Course Type: Self-Paced Online Learning Accreditation: AQA Qualification: Nationally Recognised Qualification Study Materials: High-Quality E-Learning Study Materials Certificate: Certificate upon passing the official exam Access: 1 Year Access Tutor Support- Paid Tutor Support Customer Support: 24/7 live chat available What you will learn from this course? Upon successful completion of this GCSE English Language Course, you will be able to: Develop the skill of reading a variety of excellent literature swiftly, clearly, and with connections between the books, you are reading. Acquire the skills necessary to read critically, then use what you learn from in-depth reading to improve and inform your own writing. Understand how to properly use standard English while writing in a concise and persuasive way. Develop key skills to spell, punctuate, and apply grammar correctly Become familiar with grammatical concepts, spoken and written language conventions, and a wide vocabulary, and put these abilities to use GCSE English Language Course This GCSE English Language Course is accredited by AQA and regulated by Ofqual, making it a nationally recognised credential that will improve your CV and set you apart from the competition. Through this course, you will improve your speaking, listening, reading, and writing abilities, which will aid you in showcasing a strong command of the language. Towards the end of this course, You will have the knowledge and abilities necessary to succeed in your official exam and land a job in the relevant field. Who is this Course for? The following individuals may benefit from this GCSE English Language Course: University and job applicants Anyone who wants to receive a higher grade in their GCSE Engish Anyone interested to build a career in this sector Anyone planning to pursue English language at University or A level Anyone trying to increase their chances of landing a job Anyone who wants to work in the publishing, journalism, or teaching fields Anyone who desires to develop their English Communication skills Whether you are a complete beginner or an aspiring professional, this course will provide you with the necessary skills and professional competence, and open your doors to a wide number of professions within your chosen sector. Eligibility Requirements This GCSE English Language Course has no academic prerequisites and is open to students from all academic disciplines. You must, however, first complete an initial assessment as well as a diagnostic evaluation in order for us to ascertain your progression level and discover any weak areas before we can create an individualised learning plan for you. All course learning resources will be made available to you after successfully completing this assessment, and you will have a year to view them at your convenience. Career Path This GCSE English Language Course will provide you with a fresh opportunity to enter the relevant job market and successfully escalate to advanced vocational study. Additionally, you will be able to advance your career, increase your level of competition in your chosen field, and highlight these skills on your resume. Assessment Procedure Students must complete a variety of interactive online examinations at the conclusion of each module to evaluate the understanding and skills they are learning in this GCSE English Language Course. These assessments also determine if students can demonstrate these skills effectively. At the end of the module, you can also keep track of your progress and regularly check your score. Upon successfully completing this course, you can schedule your official exam by contacting us at [email protected]. There are two paper-based assessments and a spoken language task in the AQA-accredited GCSE English Language. Assessment Format of Paper 1 Concepts covered: Reading (literature fiction text) and writing (descriptive or narrative writing) Exam time: 1 Hour 45 minutes written exam Total marks: 80 Total weight: 50% of GCSE Question format: Short question, long question and extended writing question Assessment Format of Paper 2 Concepts covered: Reading (nonfiction text and literature fiction text) and writing (presenting a viewpoint) Exam time: 1 Hour 45 minutes written exam Total marks: 80 Total weight: 50% of GCSE Question format: Short question, long question and extended writing question Non- Assessment âSpoken Languageâ Section Students will have the opportunity to showcase their speaking and listening abilities during this assessment. Skills tested: Presenting, responding to questions and feedback, and use of Standard English Total Marks: Marked by the teacher Total Weight: separate endorsement (0% weighting of GCSE) This GCSE English Language Course - AQA English Language Online is accredited by AQA and regulated by Ofqual. What is AQA? AQA, a well-known awarding body, sets standards, administers exams at the GCSE, AS, and A Level in a variety of areas, and grants qualifications to peruse a desired career. More than half of the GCSE and A-level exams that are taken and graded in the UK each year are administered by AQA. Additionally, employers and institutions all across the world highly respect these credentials. Benefits of AQA This qualification is recognised in the UK and across the world It is valued by employers all over the world Improve your employment prospects Boost your job satisfaction Promotes career advancement Enhances your CV Provides you with a competitive edge in the job market What is Ofqual? Qualifications, exams, and assessments are governed in England by Ofqual (The Office of Qualifications and Examinations Regulation). They are in charge of identifying the knowledge, skills, and understanding that students have displayed, and also ensuring that all the assessments and exams reveal what a student has accomplished. Benefits of Ofqual Regulation This regulation is valued internationally Created in accordance with specific national standards Designed in collaboration with industry to guarantee their suitability. Accurately reflect the knowledge, abilities, and understanding that the learners have shown. Candidates who have obtained a qualification that is governed by Ofqual are seen with greater confidence by employers. Course Curriculum Paper 1: Explorations in Creative Reading and Writing Reading Reading Identify Explicit Information Q1 - Identify Explicit Information 00:00:00 Assessment - Identify Explicit Information 00:10:00 Analysing Writers' Use of Language 1) Analysing a writer_s use of language 00:00:00 2) Further examples of analysing a writer_s use of language 00:00:00 Assessment - Analysing Writers' Use of Language 00:24:00 Structure 1) What do we mean by structure 00:00:00 2) How writers use structure 00:00:00 3) An example of structure in action 00:00:00 Assessment - Structure 00:24:00 Evaluating Writers' Methods 1) Evaluating a writer_s methods 00:00:00 Assessment - Evaluating Writers' Methods 00:16:00 Writing 1) Descriptive Writing - The Basic Techniques 00:00:00 2) Writing a description suggested by a picture 00:00:00 3) Creating a good story opening 00:00:00 Assessment - Writing 00:10:00 Paper 2: Writers' Viewpoints and Perspectives Reading Identify and Interpret Explicit and Implicit Information and Ideas Q1 - Identify Explicit and Implicit Evidence 00:12:00 Assessment - Identify and Interpret Explicit and Implicit Information and Ideas 00:06:00 Synthesising and Summarising 1) Synthesising and Summarising 00:11:00 Assessment - Synthesising and Summarising 00:10:00 How does the Writer use Language? How Does the Writer Use Language 00:11:00 Assessment - How does the Writer use Language 00:14:00 Comparing Viewpoints and Perspectives Comparing Viewpoints and Perspectives 1 00:11:00 Comparing Viewpoints and Perspectives 2 00:11:00 Comparing Viewpoints and Perspectives 3 00:11:00 Assessment - Comparing Viewpoints and Perspectives 00:10:00 Writing Assessment - Writing 00:20:00 Feedback Feedback 00:00:00 06) Writing Example and Revision Tasks for Question 5 00:13:00 01) Different writing tasks for Question 5 00:00:00 02) Writing to Argue or Persuade 00:00:00 03) Planning and Writing an Article 00:11:00 Writing a Speech and Expressing Opinions 00:11:00

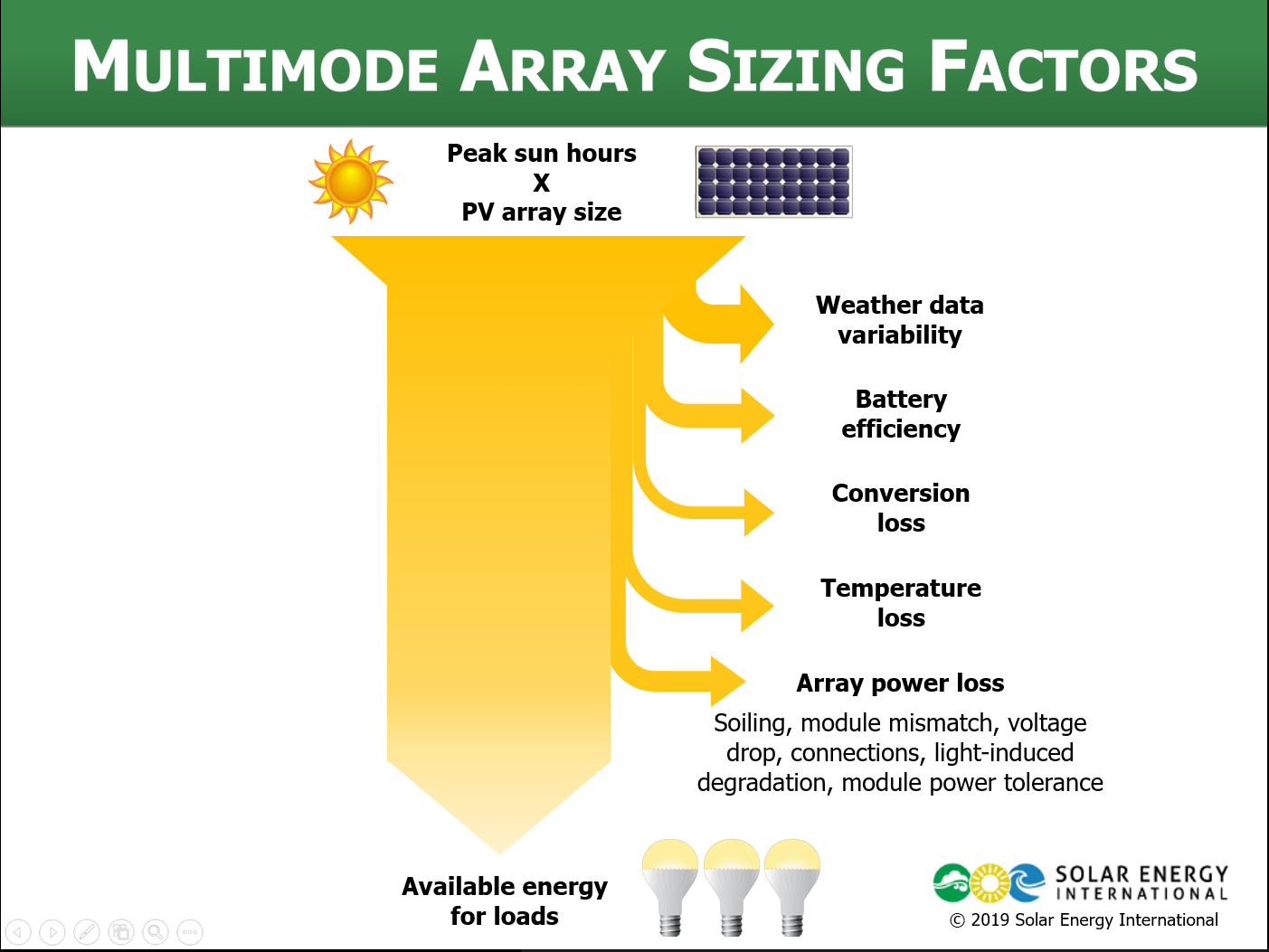
PVOL303: Solar Training - Advanced PV Multimode and Microgrid Design (Battery-Based) - Online
By Solar Energy International (SEI)
Define multimode system terminology Describe goals and applications of multimode systems Detail basic component layouts of multimode systems Define microgrid systems and diagram component layouts for microgrid applications List applications for multimode systems Distinguish between back-up and self-consumption use cases Examine daily and annual data to perform a load analysis Review battery bank sizing Identify PV array sizing methods and variables for multimode systems Calculate minimum PV array size to meet load requirements Calculate what percentage of overall annual consumption will be offset by selected PV array size Analyze data required to specify a multimode inverter Differentiate between sizing considerations for internal and external AC connections Describe various configurations for stacking and clustering multiple inverters Describe when and why advanced inverter functions are used Discuss the equipment and designs needed for advanced multimode functions Analyze each advanced multimode function List data needed to perform an accurate financial analysis of systems that use advanced multimode functions Describe factors that can affect the financial analysis of systems using advanced multimode functions Describe the National Electrical Code (NEC®) Articles that apply to the different parts of PV and energy storage systems (ESS) Identify specific requirements for ESS and systems interconnected with a primary power source List relevant building & fire codes Communicate specific requirements for workspace clearances, disconnects, & OCPD Describe PV system requirements that affect ESS installation List ESS labeling requirements Review DC coupled systems, including advantages and disadvantages Discuss MPPT charge controller operations and options Review charge controller sizing for grid-tied systems Design a DC coupled multimode PV system for a residential application Define operating modes of an AC coupled PV system while grid-connected or in island mode Explain charge regulation methods of grid-direct inverter output Review AC coupled PV system design strategies Evaluate equipment options for AC coupled multimode applications Design an AC coupled multimode PV system for a residential application Define Energy Storage System (ESS) Describe criteria for evaluating energy storage system configurations and applications Design ESS system for back-up power Describe large-scale energy storage system applications and functions; review use case examples Analyze equipment configuration options for large-scale AC and DC coupled systems Formulate questions to enable design optimization of large-scale energy storage systems Note: SEI recommends working closely with a qualified person and/or taking PV 202 for more information on conductor sizing, electrical panel specification, and grounding systems. These topics will be part of this course, but they are not the focus.