- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
2306 Courses delivered Online
Voiceover Artist and Freelance Translation - Endorsed Certificate
By Imperial Academy
Level 5 - Two Endorsed Diploma | QLS Hard Copy Certificate Included | Plus 5 CPD Courses | Lifetime Access

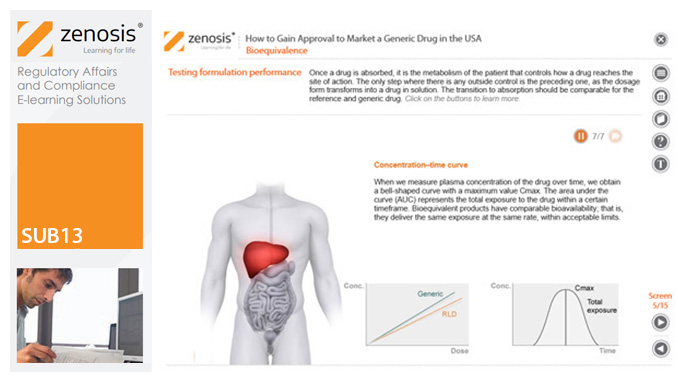
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
Level 7 Diploma in Investment Advisor Certification - QLS Endorsed
4.7(47)By Academy for Health and Fitness
Level 7 Diploma in Investment Advisor Certification – QLS Endorsed! Advance Your Finance Career, Flexible Learning, Expert Support – Enroll Today for Professional Success!

ECS Card Health & Safety Course Bromley
By MJ Electrical Training
ECS Health & Safety Course with MJ Electrical Training. Available every week, use this course to complete the application process when applying for an ECS Labourers Card.

ECS Card Health & Safety Course Dover
By MJ Electrical Training
ECS Health & Safety Course with MJ Electrical Training. Available every week, use this course to complete the application process when applying for an ECS Labourers Card.

ECS Card Health & Safety Course Liverpool
By MJ Electrical Training
ECS Health & Safety Course with MJ Electrical Training. Available every week, use this course to complete the application process when applying for an ECS Labourers Card.

ECS Card Health & Safety Course Newcastle
By MJ Electrical Training
ECS Health & Safety Course with MJ Electrical Training. Available every week, use this course to complete the application process when applying for an ECS Labourers Card.

ECS Card Health & Safety Course Kent
By MJ Electrical Training
ECS Health & Safety Course with MJ Electrical Training. Available every week, use this course to complete the application process when applying for an ECS Labourers Card.

Car Mechanic & Car Maintenance Career Oriented Job Focused Program - Money Back Guarantee
4.9(27)By Apex Learning
Gear Up for Success: Your Roadmap to Car Mechanic Proficiency with complete Car Mechanic & Car Maintenance program. Are you ready to accelerate your career in the dynamic world of automotive engineering? Welcome to a transformative ride through our Car Mechanic & Car Maintenance program - not just a course but a roadmap to restore your career in the fast lane. This certification is your passport to a fulfilling career in a world where automotive expertise is critical. The automotive industry is booming, and opportunities abound for those with the right skills. Join us in exploring the intricacies of vehicle mechanics, hybrid technologies, Car Restoration, specialised areas like supercharger engineering and many more. Moreover, we're your dedicated partners in this exciting Car Mechanic & Car Maintenance program. Our goal isn't just to teach you; it's to support you 24/7 so you can get closer to your dream job. We're so confident with our program that we offer a 100% money-back guarantee, ensuring your complete satisfaction. Learning Outcomes Upon completion of the Car Mechanic & Car Maintenance program, you will have acquired: Electrician skills tailored for automotive applications Advanced proficiency in engine tuning techniques Expertise in chassis and suspension systems In-depth knowledge of fuel injection systems Competence in hybrid vehicle technology Understanding of supercharger and turbocharging principles Proficiency in digital circuit design for automobiles Effective inventory management skills for automotive workshops Expertise in LGV and motorbike maintenance Knowledge of fire safety measures and car restoration techniques. Time management strategies tailored explicitly for mechanics Comprehensive interview skills training to excel in job interviews Confidence in navigating the virtual job market, including video interviews Optimisation of your online presence through a professionally crafted LinkedIn profile. Courses Provided Upon Enrollment Enrol in our Car Mechanic & Car Maintenance program and gain access to 25 complete courses, including: => Course 01: Car Mechanic and Repair Training Diploma => Course 02: Large Goods Vehicle (LGV) => Course 03: Motorbike Repairing and Maintenance Diploma => Course 04: Hybrid Vehicle Expert Training => Course 05: Supercharger Automobile Engineering => Course 06: Engine Lubricant System Training - Level 4 => Course 07: Energy Saving in Electric Motors => Course 08: Car Restoration => Course 09: PUWER => Course 10: A complete course on Turbocharging => Course 11: Basic Automotive Engineering: Onboard Diagnostics => Course 12: Digital Electric Circuits & Intelligent Electrical Devices => Course 13: Mechanical Engineering => Course 14: Inventory Management Training => Course 15: Driving Instructor => Course 16: Bicycle Maintenance Course => Course 17: PAT Level 4 => Course 18: Manual Handling Training | Online Course => Course 19: Fire Safety Training => Course 20: Time Management | Online Course => Course 21: Career Development Plan Fundamentals => Course 22: CV Writing and Job Searching => Course 23: Interview Skills: Ace the Interview => Course 24: Video Job Interview for Job Seekers => Course 25: How to Create a Professional LinkedIn Profile Enrol in our highly regarded Car Mechanic & Car Maintenance program, featuring a job-relevant curriculum that ensures your skills align with employer expectations across various sectors. Don't miss this opportunity - your success story starts now! Please feel free to contact us for any query, and we will gladly assist you. Embark on a transformative journey into the complex car mechanics and maintenance sector with our comprehensive Car Mechanic & Car Maintenance program. From understanding the different aspects of engine mechanics to mastering hybrid technology, our courses cover it all, ensuring you're well-equipped for a successful career in the automotive industry. Why Choose Us? So, what sets us apart from other programs? Here are the benefits you'll experience when you join our Car Mechanic & Car Maintenance Program: Updated Materials: Stay at the forefront of the ever-evolving automotive landscape with our commitment to consistently updating course materials. In the Car Mechanic & Car Maintenance industry, staying current is vital. Our regularly updated content ensures you're ready with the latest knowledge, techniques, and industry trends. Flexible Timing: Our Car Mechanic & Car Maintenance program offers a flexible learning approach. Flexible timing allows you to tailor your learning experience to fit seamlessly into your schedule. Whether you're working a full-time job, managing family responsibilities, or pursuing other interests, our program adapts to you. No Hidden Costs: Transparency is our core principle regarding your investment in education. With our Car Mechanic & Car Maintenance program, your enrollment fee covers everything you need to succeed - from certification to comprehensive course materials. There are no hidden costs or additional expenses. Money-Back Guarantee: We stand behind the value our program brings to your educational journey. If, within the first 14 days of enrollment, you find that our Car Mechanic & Car Maintenance program doesn't align with your expectations, we offer a hassle-free money-back guarantee. Your satisfaction is our priority, and this guarantee reflects our confidence in the quality and relevance of our curriculum. Lifetime Access: Education is a lifelong journey, as is your access to our Car Mechanic & Car Maintenance program. Enjoy lifetime access to our complete course materials and updates, ensuring that you remain connected with the latest advancements in automotive technology. 24/7 Support: We understand that your learning journey is more than just a course. Our dedicated support team is available around the clock, 24/7, to assist you at every step of your learning experience. Whether you have questions about course content, need clarification on a concept, or require technical assistance, our support team is just a message away. CPD 250 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Car Mechanic & Car Maintenance program is for individuals who are: Seeking a career in automotive engineering Interested in understanding car mechanics and maintenance Looking to enhance their knowledge of hybrid and electric vehicles Aspiring to work in LGV and motorbike maintenance Requirements No experience is required for the Car Mechanic & Car Maintenance program. Just enrol and start learning - we'll guide you every step of the way. Career path Upon completion of the Car Mechanic & Car Maintenance program, you will get edges in many professions, including: Automotive Technician: £25,000 - £35,000 Electric Vehicle Specialist: £30,000 - £40,000 Motorbike Mechanic: £20,000 - £30,000 Car Restoration Specialist: £25,000 - £35,000 Driving Instructor: £20,000 - £30,000 Bicycle Maintenance Technician: £18,000 - £25,000 Certificates CPD Accredited (e-Certificate) Digital certificate - Included CPD Accredited (Hard Copy Certificate) Hard copy certificate - Included e-Transcript Digital certificate - Included Hard Copy Transcript Hard copy certificate - Included Student ID Card Digital certificate - Included

Level 5 Diploma Agile Scrum Master Certification - QLS Endorsed
By Kingston Open College
QLS Endorsed + CPD QS Accredited - Dual Certification | Instant Access | 24/7 Tutor Support | All-Inclusive Cost
