- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Courses delivered Online
We couldn't find any listings for your search. Explore our online options and related educators below to see if they help you.
Know someone teaching this? Help them become an Educator on Cademy.
Online Options
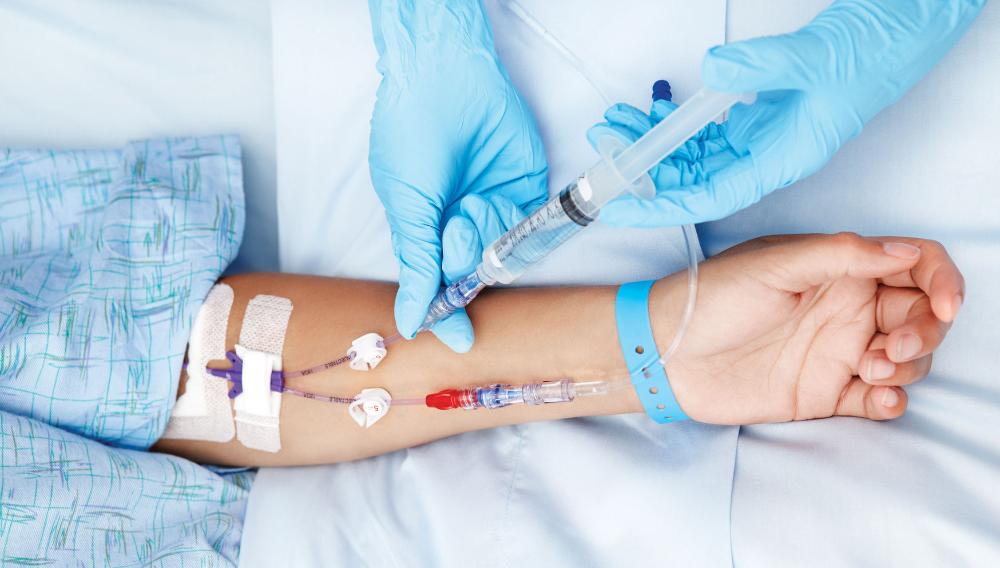
Show all 1073Learn how to cannulate ... Nationally Recognised Qualification OCN Accredited - Level 3 (advanced) - Ireland Level 5 CPD Accredited - The CPD Certification Service Classroom or Virtual Classroom options Covers all steps for cannulating in arm or hand Practise on artificial arms and fake blood! Essential qualification for all IV therapies Phlebotomy training desirable but not essential Basic understanding of English language required OPEN TO ALL APPLICANTS
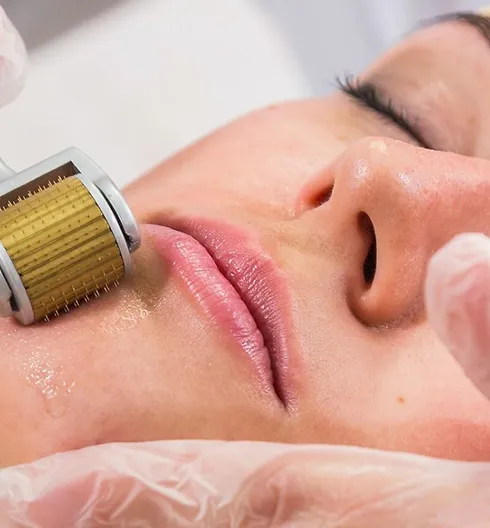
Platelet-rich Plasma (PRP) treatments Nationally Recognised Qualification No previous experience or qualifications needed Open College Network Accreditation Level 4 (as required for minimally invasive procedures) Covers standards set by HEE Employed (salon) or Self-Employed opportunities Basic understanding of English language required OPEN TO ALL APPLICANTS
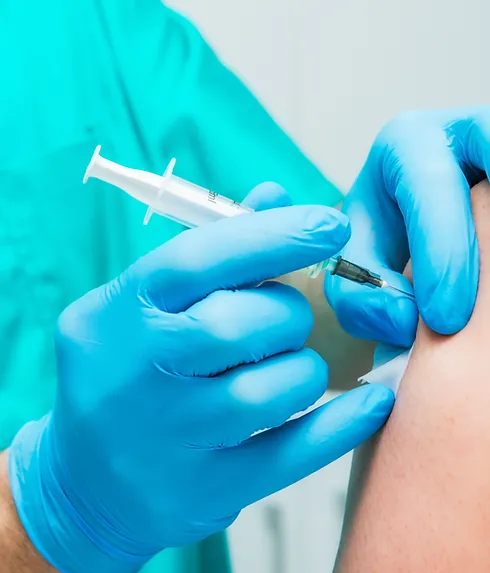
Learn how to administer vaccines or injections ... Nationally Recognised Qualification Includes IM, ID and Sub-Cut Injection methods OCN Accredited - Level 4 (Foundation Degree - FDSc) Covers all steps to safely perform a vaccination Use same techniques and skills for aesthetic therapies Includes B12, Vitamin C and other treatments Essential qualification for all injections Basic understanding of English language required OPEN TO ALL APPLICANTS
IMPORT PROCEDURES & DOCUMENTATION
By Export Unlocked Limited
This course covers import documentation and procedures, commercial considerations, the importance of your purchase order, calculating VAT and duty, and how to reduce import customs clearance delays.

IMPORTANCE OF INTERNATIONAL DOCUMENTATION
By Export Unlocked Limited
This module aims to develop knowledge and understanding of the importance of customs documentation in the world of international trade to ensure both parties clearly understand the documentation that will be required in the import or export of goods or services.

EXPORT PROCEDURES & DOCUMENTATION
By Export Unlocked Limited
International markets offer huge Export opportunities for UK businesses. Finding and developing new markets for products is a hugely valuable avenue for expansion and in some sectors in particular, global demand for British brands and products makes international trade an excellent means of growth.

GMP01d - Documentation
By Zenosis
Comprehensive documentation of procedures, formulas, work instructions, and specifications, and thorough recording of batch data, are fundamental requirements of GMP. In this short course we explain why documentation is so important, identify different types of document required, and set out some simple rules for recording and correcting data.
GMP02: Good Documentation Practice
By Zenosis
Good Manufacturing Practice (GMP) for medicinal products relies on documentation. Good Documentation Practice (GDocP) is that part of GMP that applies to the creation, maintenance, use, and retention of documents to provide assurance of the quality of products.
Documentation & Record Keeping for HCAs
By M&K Update Ltd
This session covers the legal aspects of documentation and record keeping in healthcare for Healthcare Assistants.

