- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7109 Courses in Glasgow delivered On Demand
***24 Hour Limited Time Flash Sale*** Pharmacy Skills, Medicine Management & Medical Law Admission Gifts FREE PDF & Hard Copy Certificate| PDF Transcripts| FREE Student ID| Assessment| Lifetime Access| Enrolment Letter Begin your journey towards a rewarding and successful career by enrolling in our all-inclusive bundle of 8 Pharmacy Skills, Medicine Management & Medical Law courses. At UKHF Online, we have carefully selected and combined these courses to equip you with the vital skills and knowledge necessary to thrive in Pharmacy Skills, Medicine Management & Medical Law. Whether you're a student, recent graduate, or job seeker, our Pharmacy Skills, Medicine Management & Medical Law bundle is designed to enhance your CV, impress potential employers, and set you apart from the competition. Key Features of the Pharmacy Skills, Medicine Management & Medical Law Bundle: 3 QLS-Endorsed Courses: We proudly offer 3 QLS-endorsed courses within our Pharmacy Skills, Medicine Management & Medical Law bundle, providing you with industry-recognized qualifications. Plus, you'll receive a free hardcopy certificate for each of these courses. QLS Course 01: Pharmacy Skills QLS Course 02: Medical Law QLS Course 03: Safe Handling of Medicines 5 CPD QS Accredited Courses: Additionally, our bundle includes 5 relevant CPD QS accredited courses, ensuring that you stay up-to-date with the latest industry standards and practices. Course 01: Phlebotomist Training Course 02: Medical & Clinical Administration Diploma Course 03: Clinical Observation Skills for Carers Course 04: Nurse Prescribing Diploma Course 05: Emergency Medicine - Paramedicine In Addition, you'll get Five Career Boosting Courses absolutely FREE with this Bundle. Course 01: Professional CV Writing Course 02: Job Search Skills Course 03: Self Esteem & Confidence Building Course 04: Professional Diploma in Stress Management Course 05: Complete Communication Skills Master Class Convenient Online Learning: Our Pharmacy Skills, Medicine Management & Medical Law courses are accessible online, allowing you to learn at your own pace and from the comfort of your own home. Learning Outcomes of the Pharmacy Skills, Medicine Management & Medical Law Bundle: Master the foundational principles and techniques of Pharmacy Skills, Medicine Management & Medical Law. Develop advanced proficiency in Pharmacy Skills, Medicine Management & Medical Law methodologies and strategies. Acquire in-depth knowledge of the latest trends and advancements in Pharmacy Skills, Medicine Management & Medical Law. Enhance your problem-solving and critical thinking abilities within the context of Pharmacy Skills, Medicine Management & Medical Law. Cultivate strong communication and collaboration skills essential for success in Pharmacy Skills, Medicine Management & Medical Law. The Pharmacy Skills, Medicine Management & Medical Law bundle is a comprehensive collection of courses that have been meticulously designed to provide you with a well-rounded education in Pharmacy Skills, Medicine Management & Medical Law. With a combination of 3 QLS-endorsed courses and 5 CPD QS-accredited courses, this bundle offers you the perfect balance of essential knowledge and valuable skills. What's more, we are proud to offer free hardcopy certificates for each course within the Pharmacy Skills, Medicine Management & Medical Law bundle, giving you the recognition you deserve. CPD 250 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This bundle is ideal for: Individuals interested in pursuing a career in the pharmaceutical industry Professionals in the healthcare industry looking to enhance their knowledge and skills Individuals who want to expand their knowledge and stay updated on industry trends Anyone interested in learning about pharmacy skills, medicine management, and medical law. Career path This bundle will be beneficial to anybody planning to start a career as: Pharmacy Technician: £20,000 - £28,000 Clinical Pharmacist: £36,000 - £80,000 Nurse Prescriber: £29,000 - £53,000 Medical Administrator: £18,000 - £30,000 Phlebotomist: £17,000 - £23,000 Emergency Medical Technician: £18,000 - £31,000 Certificates Digital certificate Digital certificate - Included Hard copy certificate Hard copy certificate - Included

VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

VAL05: Equipment Cleaning Validation
By Zenosis
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
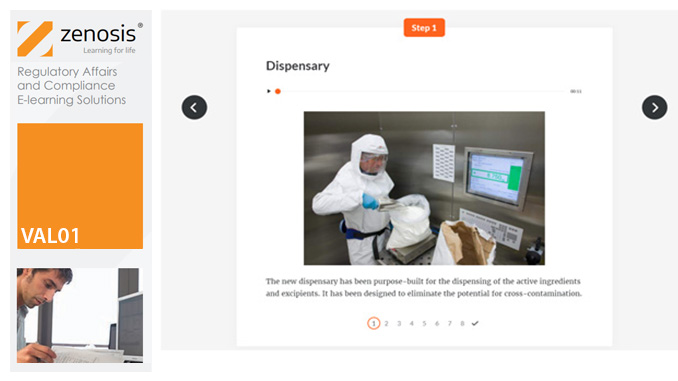
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
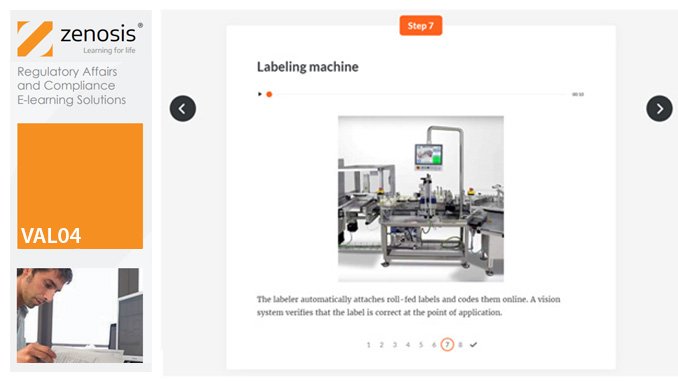
VAL04: Operational and Performance Qualification
By Zenosis
Having undergone Installation Qualification, before equipment can be used routinely in production, it needs to undergo Operational Qualification (OQ) and Performance Qualification (PQ). This module describes OQ and PQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
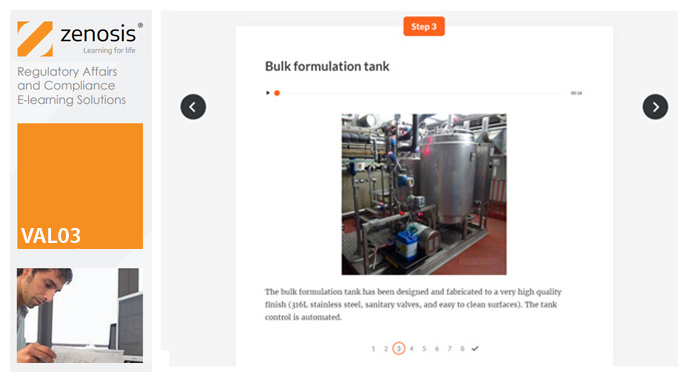
VAL03: Commissioning and Installation Qualification
By Zenosis
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
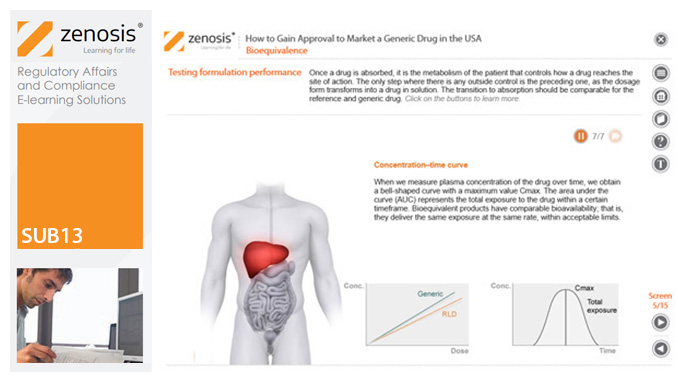
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.