- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
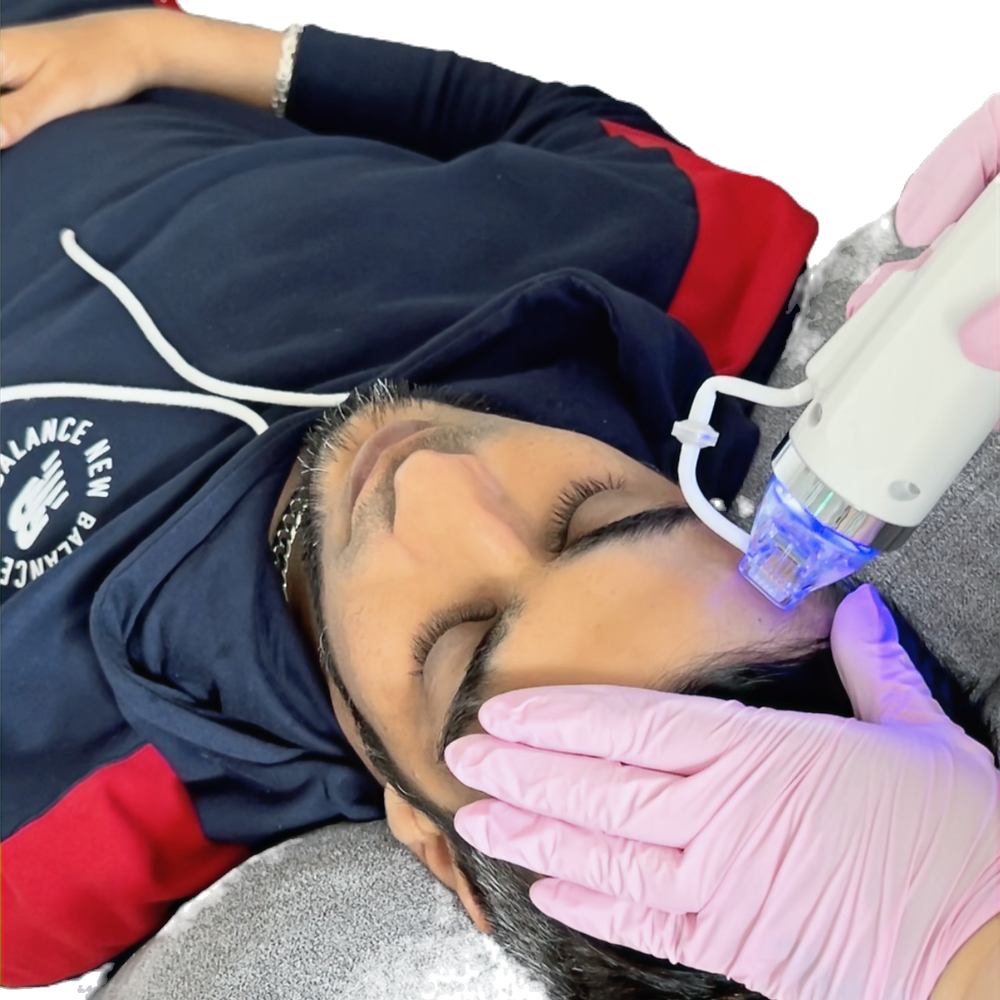
Face Online Fractional RF Microneedling- Secret microneedling
By KBH Training Academy
RF Microneedling Radiofrequency is currently the best available technology for non-invasive, non-surgical skin tightening and cellulite removal. Radiofrequency treatments are used in aesthetics to deliver intense heat to tissues deeper than the epidermis (the outermost layer of the skin), with the aim to boost collagen production, circulation and (optionally, depending on treatment depth) fat release from those tissues. Course Content: Legislations Contraindications Anatomy and physiology Health and Safety Fitzpatric What is microneedling Machines Benefits Client care Pricing Consultation Treatment protocol Difference between body MFR and SFR Aftercare Certificate You will receive an end-of-course certificate which is accredited by the cpd group and allows you to work on public How do Online Courses work? http://www.kbhtrainingacademy.com/online-courses Are there any prerequisite? You should hold A&P Level 3 qualification Duration of Course? You will have 3 months to complete the course before it expires. Will I require a model? Yes, 1 case of study is required. Please note, you will be required to have a machine to complete the training. Are there video tutorials? Yes, you will have links to YouTube and training videos to watch the treatment being performed.
Parenting Course
By Ebiere Parent Coach
Discover what is really behind your child's emotions; Delve deeper into your past and how it is affecting your parenting Discipline effectively to empower your child; Raise an Authentic and Resilient child Strengthen your relationship with your child

Node.js API Masterclass with Express and MongoDB
By Packt
Create a real-world backend for a Bootcamp directory app

20 Web Projects with Vanilla JavaScript
By Packt
Build 20 mini frontend projects from scratch with HTML5, CSS, and JavaScript

Easy Statistics: Linear Regression
By Packt
This course covers the fundamental topics of statistical methodology, linear regression, and ordinary least squares that every statistician needs to know.

ICT01: Compliance with Regulation 21 CFR Part 11 on Electronic Records and Electronic Signatures
By Zenosis
21CFR11 applies to records that are required to be submitted to the FDA, or that are subject to FDA inspection, and that are in electronic form – that is, as computer files. It applies to all computer systems used to create, modify, maintain, archive, retrieve, or transmit such records – from a humble spreadsheet program to a complex information management system.

PKPD02: Conducting Pharmacokinetic and Pharmacodynamic Studies
By Zenosis
This module extends the learner’s understanding of pharmacokinetic and pharmacodynamic studies from the basics described in our companion module PKPD01, An Introduction to Pharmacokinetics and Pharmacodynamics in Drug Development and Registration. It provides detail on a variety of aspects of such studies: design, sampling, data analysis, research in special populations, and bioequivalence testing.

SUB04: Preparing Submissions in the Common Technical Document (CTD) Format
By Zenosis
The CTD is the internationally recognised standard format for submissions to medicines regulatory authorities. In the European Economic Area, the USA and Canada, the CTD, in its electronic format (eCTD), is mandatory for all applications for marketing approval and all subsequent related submissions. The CTD is accepted in many other countries, being mandatory for new prescription medicines in some. This module explains the rationale for the CTD and provides guidance on its structure and format and the ways in which it is used.

PV04: Signal Detection and Management in Pharmacovigilance
By Zenosis
This module provides a guide to signal detection and management for approved products. The subject is presented as a process comprising four stages: signal detection, signal validation, signal analysis and prioritisation, and risk assessment and minimisation.

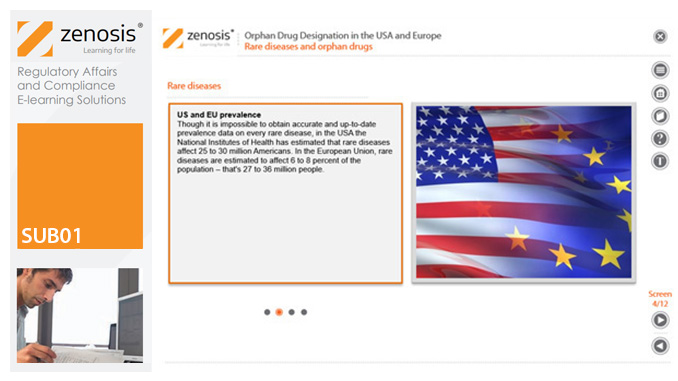
SUB01: Orphan Drug Designation in the USA and Europe
By Zenosis
Medicines for the prevention, diagnosis, or treatment of rare diseases have become known as ‘orphan drugs’ because of their commercial unattractiveness. Development of such products is successfully encouraged through incentives offered by regulatory authorities. To qualify for important incentives, the sponsor of a drug must gain ‘orphan designation’ for its use in an indication. This module describes the requirements for orphan designation and how to apply for it in the USA and the European Economic Area.