- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
883 Courses delivered Live Online
Screen Acting Weekly with Showreel
By Actors Studio
With Actress, Acting and Audition Coach Sarah Hannah Introduction Join our internationally recognised Screen Acting Weekly Course and develop your technical performance skills and character understanding over 5 Weekly Evening Sessions before applying your new skills on a professional set at Pinewood Studios as you participate in filming two showreel scenes. Over weekly sessions, the Director will use practical techniques to build your confidence in performance and help you master the technical requirements to be a successful screen actor. You will focus on the importance of character preparations, how to portray emotion, comedy and drama on camera in addition to career advice before shooting a professional acting showreel. Upon completion of the Screen Acting Weekly course, you will emerge with a heightened sense of assurance and confidence in your screen acting abilities and the tools to progress with your acting career, whether you’re new to the industry or a Drama School Graduate looking for more acting opportunities in TV & Film. Meet Your Tutor Sarah Hannah Acting Coach Sarah Hannah is a highly experienced Professional Actress, Acting and Audition Coach. Bringing her extensive experience of working on stage and on screen, Sarah will focus on enhancing your presence on camera and cover the technical skills required of a professional screen actor. Course Outline The course led by Acting Coach Sarah Hannah is held at The Audition House, Central London, and the first 5 weekly sessions run for 3 hours (6-9pm). There are 3 additional showreel filming sessions at Pinewood Studios. You will be required for 2 out of the 3 filming sessions. We film during the day and you will be given a specific filming time and will not be required all day. As this course is inclusive of all levels of ability, it offers a comprehensive overview of screen acting skills, starting from the basics and progressing to more advanced concepts. It will include resources, material and instructional stratergies that cater to beginners while also offering challenges for those with more advanced skills. Booking Options Select a course date and add to cart to proceed with payment. This course has limited availability and spaces are first come first served. Please read our Terms and Conditions before proceeding with your booking. £750.00 Weds 24th April-Thurs 30th May 2024 Lesson 1: Wednesday 24th April 2024 – 6-9pm Central London Lesson 2: Wednesday 1st May 2024 – 6-9pm Central London Lesson 3: Wednesday 8th May 2024– 6-9pm Central London Lesson 4: Wednesday 15th May 2024 – 6-9pm Central London Lesson 5: Wednesday 22nd May 2024 – 6-9pm Central London Filming Sessions: Tuesday 28th, Wednesday 29th and Thursday 30th May 2024 at Pinewood Studios. You are required for 2 of the 3 filming sessions. Weds 18th Sept-Thurs 24th Oct 2024 Lesson 1: Wednesday 18th September 2024 – 6-9pm Central London Lesson 2: Wednesday 25th September 2024 – 6-9pm Central London Lesson 3: Wednesday 2nd October 2024– 6-9pm Central London Lesson 4: Wednesday 9th October 2024 – 6-9pm Central London Lesson 5: Wednesday 16th October 2024 – 6-9pm Central London Filming Sessions: Tuesday 22nd, Wednesday 23rd and Thursday 24th October 2024 at Pinewood Studios. You are required for 2 of the 3 filming sessions. Additional Information Features Practical acting training for screen Shoot two scenes for your professional acting showreel with our Award Winning Crew Suitable if you are (17 yrs+) and committed to improving your screen acting skills Attracts a varied age range from 17 yrs+. International Students Welcome The course is taught in English, so it is important that you have proficient English language skills Small Class Size Learning Outcomes Learn techniques to effectively analyse a Film/TV Script Create truthful and inspirational characters Build confidence performing in-front of camera Understanding the workflow of a busy film set Audition preparation Career advice and direction – how to market yourself Course Location Training Sessions (1-5) 6pm – 9pmThe Audition House, 129A Whitfield Street, Fitzrovia, London, W1T 5EQ The Audition House is a two minute walk from Warren Street tube and 5 minutes from Euston Station Filming Sessions (6-8) Between 8am and 4pmActors Studio, Pinewood Studios, Pinewood Road, Iver Heath, Buckinghamshire, SL0 0NH Free Parking available at Pinewood Studios Free frequent shuttle bus service from London Uxbridge Tube Station, Slough and Gerrards Cross. Transfer from station to Pinewood Studios – 10 minutes Canteen and Coffee shops on site Excellent transport links from London

Introduction to Yoga and Beneficial Practical Parapsychology Course
By Integral Studies Academy
Immerse yourself in the ancient practice of yoga, a transformative journey that extends far beyond the mere physical postures. Our comprehensive yoga sessions are meticulously curated and led by seasoned professionals, offering a serene sanctuary tailored for the demands of modern-day professionals yearning for holistic balance and wellness. Delve into innate human abilities such as intuition, telepathy, clairvoyance, lucid dreaming, and energy healing. Uncover these dormant gifts existing within and enjoy awakening them fully.

Recruiting Talent and Termination Procedure
By NextGen Learning
Recruiting Talent and Termination Procedure Course Overview: This comprehensive course on "Recruiting Talent and Termination Procedure" provides an in-depth understanding of the recruitment process, effective interviewing methods, and employee termination procedures. Learners will explore a wide range of topics, from creating effective recruitment strategies to managing employee terminations in a legal and ethical manner. The course offers practical value by equipping learners with the necessary knowledge and skills to excel in HR roles and manage critical HR processes. On completion, learners will be prepared to contribute effectively to recruitment and employee management within an organisation. Course Description: This course covers the entire recruitment lifecycle, including the development of recruitment models, selection methods, and the nuances of human resource management. Key topics include virtual interviewing techniques, employee on-boarding strategies, and the legal and procedural aspects of employee termination. Through a structured learning experience, learners will develop skills in recruitment planning, interview preparation, and managing the complexities of terminating employment. With a focus on the latest HR practices, the course ensures learners are prepared to handle recruitment and termination tasks with professionalism and confidence. Recruiting Talent and Termination Procedure Curriculum: Module 01: Introduction to Recruitment Process Model Module 02: Recruitment Methods Module 03: Human Resource Management Module 04: Key Skills and Issues in Recruitment Function Module 05: Virtual Interviewing Module 06: Employee On-Boarding Module 07: Introduction to The Employee Termination Module 08: The Employee Termination Procedure Module 09: Employee Termination Letter and Guide (See full curriculum) Who is this course for? Individuals seeking to enhance their understanding of recruitment and employee management. Professionals aiming to advance their career in HR or recruitment. Beginners with an interest in human resource management. HR practitioners seeking to update their skills in recruitment and termination processes. Career Path: HR Manager Recruitment Consultant Talent Acquisition Specialist Employee Relations Officer HR Coordinator HR Director

Introduction to Good Manufacturing Practice
By Research Quality Association
Course Information This course offers foundational guidance and practical support tailored for individuals operating within Good Manufacturing Practice (GMP) frameworks. Explore the fundamental prerequisites of a pharmaceutical quality system (PQS) and delve into the application of quality risk management (QRM) principles, aligning with current regulations and guidance. Gain insights into pivotal aspects such as requirements, roles, and responsibilities, encompassing change control, document management, and key documentation essential for effective implementation of GMP with a focus on regulatory inspections and common findings. Is this course for you? Ideal for professionals engaged in GMP across various sectors, including: Research and Development (R&D) Contract Manufacturing Organisations Manufacturing Units Quality Control (QC) Laboratories Auditing Roles. What will you learn? Event objectives - by the end of the course, delegates shall: Have an awareness of the basic requirements of GMP Be aware of UK and EU GMP Rules and Guidance and relevant publications Understand the roles and responsibilities associated with GMP Be able to contribute to and maintain quality documentation Have a basic understanding of product lifecycle and manufacturing Understand the requirements of GMP in the QC laboratory context Have a basic understanding of risk management and mitigation principles Understand the need for quality systems and quality assurance activities Be aware of common regulatory findings. Learning outcomes: delegates will be able to: Implement their role within GMP with confidence and knowledge of the principle requirements Contribute effectively to the GMP quality system and their organisation’s compliance Comprehend where their organisation’s activities sit within the larger GMP arena Know where to seek further information within the published rules and guidance, UK Legislation, European Commission Directives, ICH Guidance and other relevant publications, as well as via the internet. Tutors Tutors will be comprised of (click the photos for biographies): Louise Handy Director, Handy Consulting Ltd Programme Please note timings may be subject to alteration. Day 1 09:30 Introductions and Scope of the Course Understand the group requirements and the tutor's background and experience. 09:45 Background and Regulatory Environment Setting the scene, understanding the context, key legislation. 10:30 Principles of GMP Key points and requirements. 11:15 Break 11:30 Personnel and Responsibilities Management and staff, duties and accountabilities. 12:00 Overview of GMP Manufacturing Basics of the product life cycle. 12:30 Lunch 13:15 Risk Management Workshop Practical exploration of risk and mitigation activities. 14:30 QC Laboratories Activities and practicalities. 15:15 Break 15:30 Compliance Quality Assurance and Self Inspection. 16:15 Question Time A chance for questions on the practicalities of GMP. 16:30 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Learn

Audit Programmes and Risk Assessment
By Research Quality Association
Course Information This one day course is designed to provide you with comprehensive guidance and practical help for when designing and implementing audit programmes. Using the guidance of ISO 19011 with reference to PV, GCP, GMP and GLP audit programmes, you will explore audit programme design, operation, review and improvement. The course will work through why audits are important and understanding the drivers behind a good audit programme. It will discuss how to identify and assess the risks in your organisation, linking them with organisational goals, using these risks as a basis for the design of a risk-based audit programme during facilitated practical workshops. Delegates will have the opportunity to consider and discuss common issues and constraints that may shape their audit programmes. By the end of the course you will have: A clear understanding of the role of audit programmes in managing compliance and of the drivers and risks behind audit programmes An understanding of the roles and responsibilities of management and personnel An appreciation of resourcing implications and auditor attributes A good insight into the practicalities and activities required for design of risk based audit programmes A comparison of your circumstances, challenges, common issues and ways to approach managing audit programmes with other delegates on the course. Tutors Tutors will be comprised of (click the photos for biographies): Louise Handy Director, Handy Consulting Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:15 Why do we Need to Audit? Exploring risk and regulation, responsibility and performance. 09:45 Discussion - Programmes, Needs, Commonalities Understanding the needs of delegates' own organisation and comparing common themes and threats. 10:00 Establishing an Audit Programme Design, objectives, risk and resources. This session discusses the consideration when designing audit programmes. 10:30 Break 10:45 Risk Management Considerations, guidance and methods for assessing and controlling risk. 11:00 Workshop 1 - Risks, Prioritisation and Control Looking at specific risks, assessing and evaluating to feed into audit programme management. 12:00 Workshop 1 - Feedback 12:30 Lunch 13:30 Putting it into Practice Resources, practicalities and challenges - the realities of auditing, including selection of auditors, ensuring practice will meet expectations and the reasons to note audit results. 13:45 Workshop 2- Designing Audit Programmes Designing audit programmes using output from risk assessment process. Challenges and flexibility. 15:00 Break 15:15 Workshop 2 - Feedback 15:30 Monitoring, Reviewing and Improving Why or when should existing processes change, understanding the implications of change or inaction and exploring how to improve the audit programme. 16:15 Panel Session This final session will address any outstanding issues raised by delegates. 16:30 Close of Course Extra Information Remote Course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Develop

PYTHON BOOTCAMP: This 12-week Python Data Analytics Data Boot Camp is designed to give you a complete skill set required by data analysts . You will be fully fluent and confident as a Python data analyst, with full understanding of Python Programming. From Data, databases, datasets, importing, cleaning, transforming, analysing to visualisation and creating awesome dashboards The course is a practical, instructor-lead program.

Good Laboratory Practice Refresher and Hot Topics
By Research Quality Association
Course Information Join us for a comprehensive refresher focusing on crucial Good Laboratory Practice (GLP) requirements, including an emphasis on data integrity, recent developments, and emerging trends gleaned from MHRA inspections. The programme dives into specific domains such as risk assessment, OECD guidance on sponsor influence, and the advisory from OECD on QA. Additionally, delegates can benefit from a dedicated GLP clinic, facilitating discussions on understanding and upholding GLP compliance. Is this course for you? This course is tailored for study directors, principal investigators, test facility management, and QA professionals seeking to refresh their knowledge and responsibilities within the GLP framework. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Registration, Welcome and Introduction 09:20 Development of Good Laboratory Practice A reminder of the history of GLP, its current scope and application, with a synopsis of current UK, European and international standards. 09:50 Roles and Responsibilities of Study Director, Test Facility Management, Principal Investigator, Test Site Management, Study Staff and QA A reminder of the roles and responsibilities with regard to the GLP management and oversight of the Test Facility and the management and control of the study, as defined by GLP. 10:30 Break 10:45 Workshop 1 Workshop 1 Roles and responsibilities 11:15 Influence of Sponsors The published OECD Position Paper No. 21 regarding Possible Influence of Sponsors on conclusions of GLP Studies is reviewed and discussed. 11:45 Data Integrity The fundamentals of data integrity according to the OECD Guidance No. 22 on Data Integrity is discussed along with the responsibilities of Study Director, Test Facility Management, and study staff in ensuring the integrity of the GLP study data. 12:30 Lunch 13:15 Quality Assurance and GLP OECD Advisory No. 23 (Revision of OECD No.4)- A walk through of the changes to the OECD Guidance on the role and activities of Quality Assurance 13:45 Quality Improvement Tools and GLP The tools that might be considered for GLP and their role and operation when used in Test Facilities- OECD Position Paper No.24 published July 2022 14:15 Workshop 2 Workshop 2 Change control 14:30 Risk Assessment How should we assess risk and how can we use the process to assist in evaluation audit findings? 15:00 Break 15:15 Current hot topics in GLP Explore the current issues in Industry and trends /types of Regulatory inspection findings 15:50 GLP Clinic An opportunity to discuss any other issues regarding understanding and maintaining GLP Compliance. 16:30 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Learn

Leading Cross-Cultural Virtual Teams: In-House Training
By IIL Europe Ltd
Leading Cross-Cultural Virtual Teams: In-House Training High-performing teams are a must in this world of intense competition and higher expectations. Global virtual teaming has become a necessity as organizations become increasingly distributed and suppliers and clients actively engage in joint projects. Teams work across geographical and organizational boundaries to deliver solutions and services to global users where distance and differences, both geographic and cultural, amplify the effect of issues and factors that are relatively straightforward when managing a team of people in the same location. This course delivers practical concepts and techniques that participants will start using immediately on their global projects. What you will Learn At the end of this program, you will be able to: Define relationships among foundational concepts (leadership and three dimensions of diversity) and explain their potential impacts on project performance Describe key components of successful project leadership and build selected Transformational Leadership skills Prepare to convert project challenges stemming from personal or cultural diversity into potential competitive advantage Implement selected best practices to meet key challenges facing virtual project teams Foster and grow an environment that supports continued success for CCVTs Foundation Concepts Basic definitions Critical success factors for leading cross-cultural virtual teams (CCVTs) A roadmap to success for leading CCVTs Leadership Excellence in Any Project Environment Leading effectively in a global environment Transformational leadership The four components of Transformational Leadership Leveraging Personal Diversity Overview of personal diversity Mind styles The theory of multiple intelligences Connecting Transformational Leadership and personal diversity Embracing Cultural Diversity Introduction to cultural intelligence The impact of culture Cultural Dimensions Theory The Culture Map Managing Virtual Diversity Overview of virtual diversity Virtual time management Virtual processes and technology Virtual leadership Creating an Environment for Success Supporting a cross-cultural virtual-team (CCVT-) friendly environment Building a foundation of trust Developing a team charter Recap and review Summary and Next Steps Personal action plan

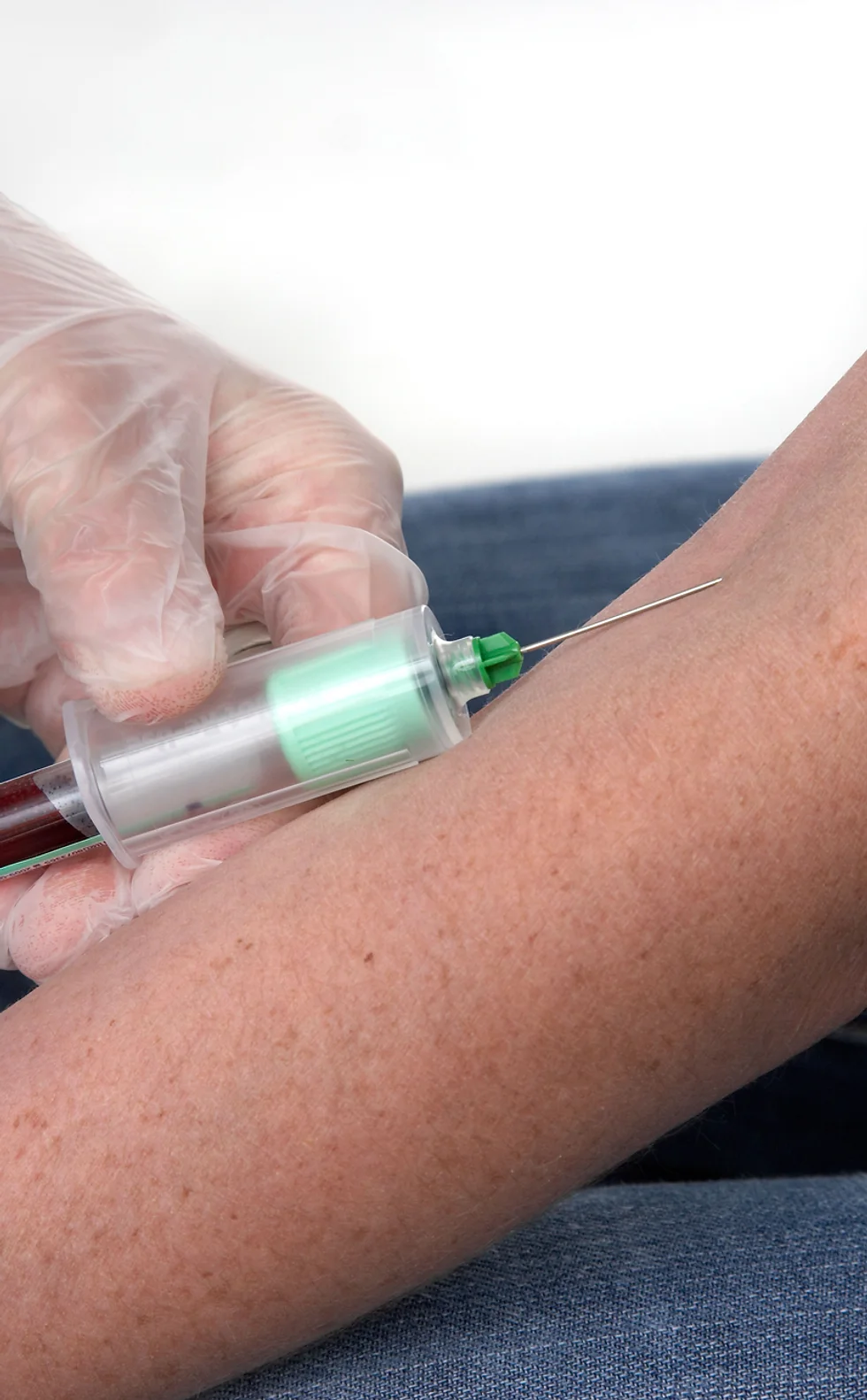
Introduction to Phlebotomy Course - Certificate Update and Renewal (GPT003R)
4.6(39)By Geopace Training
Update and renewal option for Geopace Training Certificate holders Update and renew your existing certificate ... Recommended certificate renewal after 18 months Nationally Recognised Dual Accreditations Open College Network Accredited (Advanced Level) CPD Accredited (The CPD Certification Service) Recaps phlebotomy theory incl. legislation Updates your certification and compliancy Awards cumulative credits and CPD points awarded Basic understanding of English language required Download a certificate on completion of your online course APPLICANTS ARE REQUIRED TO HAVE COMPLETED AN ACCREDITED INTRODUCTION TO PHLEBOTOMY COURSE
Kick Start Your Career with CompTIA's Data Analysis Certification - Live Classes
5.0(1)By Media Tek Training Solutions Ltd
Get job ready with CompTIA's Data Analysis Certification. Live Classes - Career Guidance - Exam Included.
