- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7055 Courses in Strabane delivered Live Online
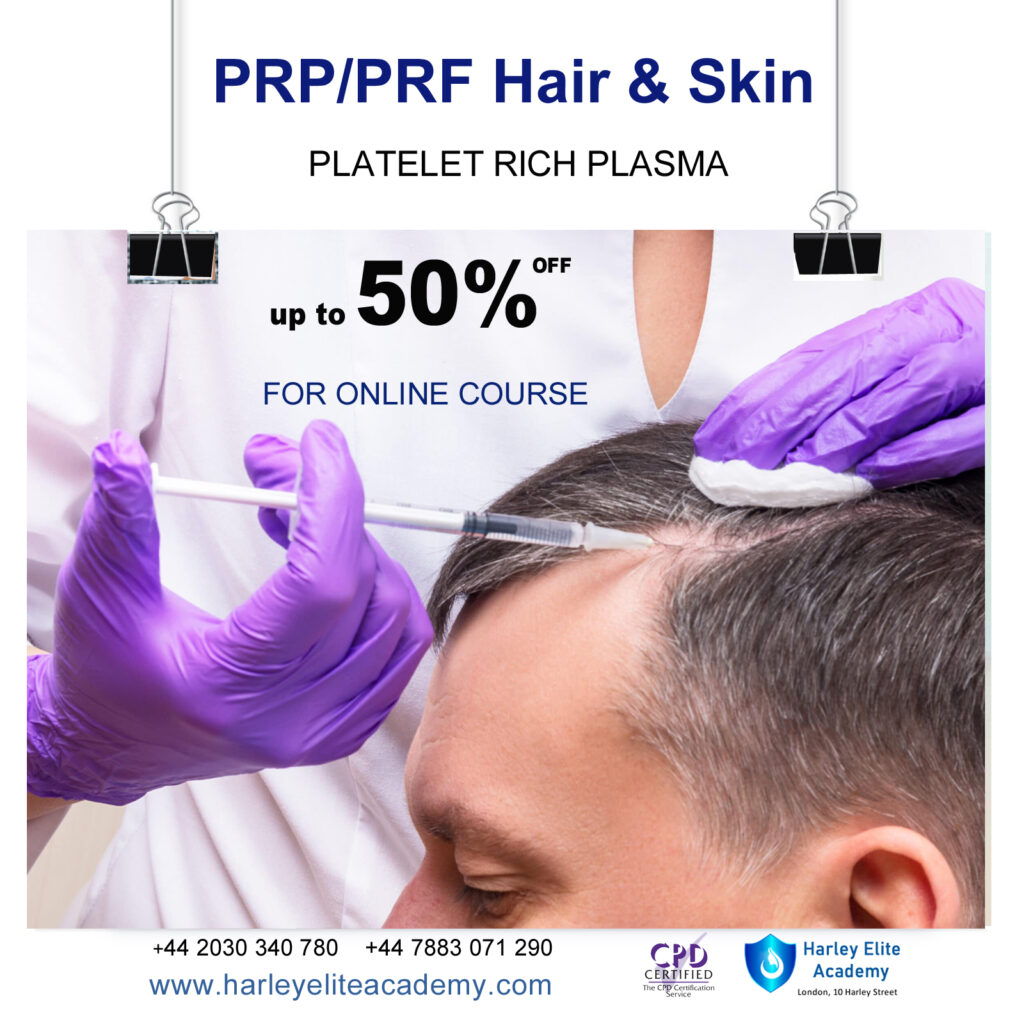
PRP / PRF SKIN & HAIR COURSE
By Harley Elite Academy (HeLa)
MASTERCLASS 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! CLINICAL PRP • Sports medicine • Traumatology • Ophthalmic • Burn trauma • Wound healing –diabetic foot • Skin grafting • Dentistry-sinus lift • Tooth implants. PRP theory & equipment: Training Online Theory will enable you to understand: Anatomy Vascular Supply, Contraindications Patient consultation Complications Management Post treatment advice Dealing with equipment A certification of training will be provided upon completion of the course. Aesthetic PRP • Skin rejuvenation • Hair restoration • Fat grafting in combination PRP • Post laser •Acne & Rosacea •Acne scar •TissueVolumisation alternative of HA fillers •Aesthetic gynecology /urology. Plathelet Rich Plasma We will cover pertinent information including mechanism of action, safety and efficacy issues, management and treatment of complications, dilution guidelines, and more. Hands on practical session – skin rejuvenation and hair loss Extraction, Preparation and Dosage Management Injection techniques – face, neck and head (hair loss); also the use of cannula Upon successful completion of the course, you will receive a certificate and title of PRP Certified Practitioner. MASTER CLASS PRP & PRF During the course we are providing . Taking blood and how to use a Centrifuge . PRP injecting techniques in face neck and décolletage hands. PRP Microneedling using a DERMAPEN. Combination treatment PRP with Mesotherapy. MECHANISM OF ACTION Platelets + Leucocytes form 3D mesh release of GF Chemo attraction and migration of macrophages and stem cells Stem cells proliferates by mitosis Stem cells undergo differentiation process BENEFIT FROM PRP TREATMENT & THERAPYExperience the advantages of PRP treatment and therapy, utilizing autologous blood with natural growth factors for disease-free and hypoallergenic benefits. Boost wound healing by regulating mitosis, proliferation, and differentiation, enhancing tissue with collagen, elastin, and hyaluronic acid. Benefit from improved tissue oxygenation, nutrition flow, and support for procedures like hair transplants, fat transfers, and skin grafts.PRP works effectively in skin rejuvenation, facial resurfacing, microneedling, and combines well with HA, PDO threads, skin boosters, peeling, or CO2 lasers. It also proves beneficial for hair restoration, showing positive results in various protocols for Androgenic alopecia and age-related hair loss.PRP where works .Skin rejuvenation-facial resurfacing.application-injection alone. Microneedling Combination with HA,Combination with PDO threads,Skin boosters , peeling or CO2 lasers Hair restoration, Multiple protocols with positive results Evidence for improvement of: Androgenic alopecia-male and females, “spot hair lost” Improvement of age related hair loss. You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (Theory), IN CLINIC (Practice) COURSE LEVEL EXPERT | Masterclass Course
Advanced Certificate in Fund Administration
By International Compliance Association
This qualification from our partner organisation CLTI will enable you to develop your knowledge of fund operations, across both accounting and administration and creates a training and education path for fund administrators where there have traditionally been limited training opportunities to evidence knowledge and support career development. Demonstrate a detailed understanding of the functions of key parties in a fund and how they meet the needs of stakeholders. Understand the lifecycle of different fund structures, including the processes involved in their closure, winding down and/or liquidation. Demonstrate awareness of the topical issues surrounding alternative investment funds, fund of funds and debt funds. Present a detailed knowledge of fund regulation and be able to interpret and apply Principal Documents, Scheme Particulars and investment restrictions. Understand the fundamentals of fund accounting and how to account for specific fund transactions. Carry out a range of advanced calculations in relation to a fund, including NAV, GAV, performance fees, and entry and exit charges. Calculate earnings per share and the total expense ratio, and use ratios to analyse a set of financial statements. Determine the impact of taxation on different fund structures and be able to carry out relevant tax calculations. This qualification covers the following topics: Fund structures, strategies and regulation Advanced find accounting and analysis

Power BI Data Analyst (PL300)
By Online Productivity Training
OVERVIEW This official Microsoft Power BI training course will teach you how to connect to data from many sources, clean and transform it using Power Query, create a data model consisting of multiple tables connected with relationships and build visualisations and reports to show the patterns in the data. The course will explore formulas created using the DAX language, including the use of advanced date intelligence calculations. Additional visualisation features including interactivity between the elements of a report page are covered as well as parameters and row-level security, which allows a report to be tailored according to who is viewing it. The course will also show how to publish reports and dashboards to a workspace on the Power BI Service. COURSE BENEFITS: Learn how to clean, transform, and load data from many sources Use database queries in Power Query to combine tables using append and merge Create and manage a data model in Power BI consisting of multiple tables connected with relationships Build Measures and other calculations in the DAX language to plot in reports Manage advanced time calculations using date tables Optimise report calculations using the Performance Analyzer Manage and share report assets to the Power BI Service Prepare for the official Microsoft PL-300 exam using Microsoft Official Courseware WHO IS THE COURSE FOR? Data Analysts with little or no experience of Power BI who wish to upgrade their knowledge to include Business Intelligence Management Consultants who need to conduct rapid analysis of their clients’ data to answer specific business questions Analysts who need to upgrade their organisation from a simple Excel or SQL-based management reporting system to a dynamic BI system Data Analysts who wish to develop organisation-wide reporting in the form of web reports or phone apps Marketers in data-intensive organisations who wish to build visually appealing, dynamic charts for their stakeholders to use COURSE OUTLINE Module 1 Getting Started With Microsoft Data Analytics Data analytics and Microsoft Getting Started with Power BI Module 2 Get Data In Power BI Get data from various data sources Optimize performance Resolve data errors Lab: Prepare Data in Power BI Desktop Module 3 Clean, Transform And Load Data In Power BI Data shaping Data profiling Enhance the data structure Lab: Load Data in Power BI Desktop Module 4 Design A Data Model In Power BI Introduction to data modelling Working with Tables Dimensions and Hierarchies Lab: Model Data in Power BI Desktop Module 5 Create Model Calculations Using DAX In Power BI Introduction to DAX Real-time Dashboards Advanced DAX Lab 1: Create DAX Calculations in Power BI Desktop, Part 1 Lab 2: Create DAX Calculations in Power BI Desktop, Part 2 Module 6 Optimize Model Performance Optimize the data model for performance Optimize DirectQuery models Module 7 Create Reports Design a Report Enhance the Report Lab 1: Design a Report in Power BI Desktop, Part 1 Lab 2: Design a Report in Power BI Desktop, Part 2 Module 8 Create Dashboards Create a Dashboard Real-time Dashboards Enhance a Dashboard Lab: Create a Power BI Dashboard Module 9 Perform Advanced Analytics Advanced analytics Data Insights through AI Visuals Lab: Perform Data Analysis in Power BI Desktop Module 10 Create And Manage Workspaces Creating Workspaces Sharing and managing assets Module 11 Manage Datasets In Power BI Parameters Datasets Module 12 Row-Level Security Security in Power BI Lab: Enforce Row-Level Security

Data Protection (GDPR) Practitioner Certificate
By CloudLearn
We are data protection specialists and this is our flagship training programme for Data Protection Officers, Data Protection Managers, Compliance Managers or anyone with a responsibility for Data Protection. The Data Protection (GDPR) Practitioner Certificate is an internationally recognised qualification, endorsed by TQUK, which is regulated by Ofqual, a UK Government department. It equips current and aspiring data protection officers and data protection managers with knowledge and skills to undertake data protection compliance activities throughout an organisation. It is a valuable course for anybody with data protection compliance responsibilities. The course takes account of the latest developments in this fast moving subject, together with the latest guidance from the ICO and includes real life, practical examples throughout. There are two versions of the course (with the same content and same trainer). The courses priced at £1200 are run by Computer Law Training and lead to the TQUK endorsed certificate. The courses priced at £1440+VAT are run in collaboration with, and are booked through, the Law Society of Scotland and, on successful completion, lead to the TQUK endorsed certificate and a 'Certified Specialist' certification from the Law Society of Scotland. Suitability - Who should attend? The training programme for Data Protection Officers, Data Protection Managers, Compliance Managers, Corporate Governance Managers or anyone with a responsibility for Data Protection. The Data Protection (GDPR) Practitioner Certificate is ideal for you if you: Are already undertaking the role of Data Protection Officer Expect to be filling the post of Data Protection Officer in their current employment Are looking for employment as a Data Protection Officer Have, or expect to have, data protection responsibilities in their organisation Need to advise others on data protection compliance Wish to be able to demonstrate verifiable practical skills and learning in this area. It is suitable for those working in: the public sector, the private sector and the third sector. In either case, it will teach participants essential data protection skills and in depth knowledge. Outcome / Qualification etc. Understand the importance of data protection legislation and compliance in the UK and beyond. Interpret key terminology of the UK GDPR and Data Protection Act 2018 (DPA) in a practical context Understand the key obligations of the UK GDPR and DPA Create appropriate policies and procedures necessary for data protection compliance Carry out a data protection audit and gap analysis Develop an action plan to address a data protection gap analysis Respond appropriately to data protection issues arising in an organisation Carry out the duties of a data protection officer Undertake accountability and risk analysis activities Training Course Content Day 1 Data Protection – history and background GDPR Overview What, really, is personal data Purposes & Legal Bases Day 2 Consent Special Categories of Personal Data Data Subject Rights Transparency Requirements Data Processors and Controllers Information Security Obligations Breach Reporting and Recording Day 3 Restricted (International) Transfers Cloud Services Accountability The Personal Data Audit & Record of Processing The “Accountability Portfolio” Data Protection by Design & Default Data Protection Impact Assessments Privacy Enhancing Technologies Data Protection Officers Direct Marketing & Cookies Day 4 Data Protection Act 2018 HR Issues Risk Frameworks Data Protection Governance Day 5 Data Protection Audit Gap Analysis Action Plan Implementation The ICO and Enforcement Brexit and the future (crystal ball!) The European Data Protection Board (EDPB) Questions Course delivery details The course is delivered on Zoom. It lasts 5 days over 3 weeks, 9.30-16.30. The advertised start date is usually a Tuesday which is the first day of the course. The course normally continues on the Thursday of that week, Tuesday and Thursday the following week and one day in the third week: 24, 26, 31 January & 2, 7 February 2023 The one-hour test to obtain the certificate is online by arrangement in the week or two following the course. The trainer for the course is Tim Musson, who has a Master of Laws degree in IT and Telecoms Law, is a Certified Information Privacy Professional (CIPP/E) and a Certified Information Privacy Technologist (CIPT).

RCN Accredited Asthma and COPD Course NORFOLK ICB ONLY
By BBO Training
'RCN Accredited Asthma and COPD' CourseThis course is a collaborative offering between your employer and BBO Training Ltd., designed to equip experienced healthcare professionals working in primary care settings with a comprehensive and holistic approach to managing patients with asthma and COPD. Even for those already holding a Diploma in either of these areas, the course has proven to be highly valuable, as indicated by positive evaluations from healthcare professionals.Course AimThe primary objective of this course is to provide qualified healthcare professionals with the necessary knowledge and skills to deliver effective asthma and COPD services in collaboration with their medical colleagues within the community healthcare setting. The course aims to raise awareness of these chronic conditions within the community, ensuring safe practice through the utilization of Patient Group Directions (PGDs) and protocols where appropriate. Participants will be clinically and theoretically prepared to establish and manage review and diagnostic clinics within their competency framework. Furthermore, the course emphasizes the clear differentiation between asthma and COPD.Clinical Practice Program and CompetenciesThroughout the course, you, along with your primary mentor, will organise and validate your clinical engagements, both under supervision and independently. By the time of your final assessment, these forms should be fully completed and ready for submission, accompanied by both your and your mentor's evaluations. Additionally, maintaining a log of all clinical hours and interactions is required, with a minimum of 36 hours.Teaching and Learning ApproachUpon completing this course, students will have the opportunity to:1. Participate in various sessions, including workshops, simulations, discussions, seminars, and tutorials, which can be conducted either virtually or in-person.2. Gain practical experience in asthma and COPD management within their local healthcare settings.3. Develop an in-depth understanding of treatments, including their composition, efficacy, indications, contraindications, and mechanisms of action, through clinical practice.4. Enhance advisory skills related to respiratory care on a broader scale.5. Attend dedicated sessions for clinical practice.6. Document a minimum of 36 hours of supervised clinical practice and maintain an ongoing reflective diary.Expected Course ContentWorkshops, Discussion Groups, Lectures, and Assessment: 40 hoursClinical Practice: A minimum of 36 hoursVisits and Private Study: 44 hoursPrivate study time is essential for reading, conducting factual research on treatments through online sources, and accessing the library for literature searches. This time will facilitate writing, referencing, completing your reflective diary, and preparing for the final assessment.AssessmentSuccessful completion of all components is required. Components 2 to 4 must be passed to complete the course. A resit option is available for any component that is not initially passed, limited to one attempt.1. Reflective Diary: This ongoing document, produced by the student throughout the course, is validated by both the student and mentor. While not directly marked, its completion is integral to your portfolio of evidence.2. Clinical Outcomes: These must be validated and passed by your mentor, with all outcomes achieved, along with a log of clinical hours.3. VIVA: A minimum pass mark of 50% is required.4. Test of Knowledge: A minimum pass mark of 50% is necessary (conducted at the end of the initial study days).Learning OutcomesUpon completion of this course, students will be capable of:1. Evaluating and showcasing clinical competence through a reflective diary/log.2. Demonstrating clinical assessment and treatment proficiency in a VIVA examination and knowledge assessment.3. Effectively educating individuals about self-management and enhancing their understanding of their condition.4. Displaying sensitivity and competence in obtaining comprehensive patient histories.5. Adapting care for diverse patient groups and their unique needs.6. Fulfilling professional responsibilities, including meticulous record-keeping and maintaining confidentiality.7. Teaching the use of various inhalers and assessment equipment.8. Developing fundamental assessment and examination techniques for respiratory conditions.9. Operating with Patient Group Directions and protocols for asthma and COPD care.10. Assessing, planning, implementing, and evaluating individual patient needs comprehensively.
Level 5 Diploma in Health & Social Care
By Egraduate College
Become a qualified Healthcare Support Worker in 6 months, 100% online with live interactive lessons and dedicated tutor support. Flexible payments.

Customer / User Research Methods
By Bunnyfoot
This one-day course introduces the field of user experience and provides an excellent entry point to our other specialised training courses. UX processes and practices have become a central component of product design, service design and web design.

Rapid Prototyping with Axure
By Bunnyfoot
This one-day course introduces the field of user experience and provides an excellent entry point to our other specialised training courses. UX processes and practices have become a central component of product design, service design and web design.

Figma Basics
By Bunnyfoot
Course description Figma is an industry leading web-based design tool that allows you to create user interfaces for any screen type or size, collaborate on those designs in real-time and build interactive prototypes suitable for user testing. Figma is a visual design tool so there’s no coding involved. Design is achieved by adding, combining and styling shapes, text and images on a canvas or ‘Frame’. We run 2 consecutive Figma training days, the second day building on the practical activities from the first. You can attend just the first day. Attending just the second day is only recommended if you’re already very comfortable with the Figma interface. Day 1: Basic Figma design and collaboration For beginners with no prior experience with Figma who want to understand the basics, be able to create their own designs, share, collaborate and even iterate on the designs of others. You will learn: How to navigate the Figma interface. How to create your own designs by: Setting up a design file. Setting up the screen type you want to design and add grids and guides. Adding and modifying basic shapes and text to create user interface elements. How to save text and colour styles for reuse. How to create reusable components. How to use the ‘auto layout’ feature to promote tidy, consistent and usable design. How to collaborate on designs by sharing screens and commenting on designs. A brief introduction to prototyping: What’s possible. What’s not. Day 2: Component variants, design systems and prototyping This session will cover the more sophisticated aspects of Figma use such as: How to use component variants to create: Interaction styles such as hover and mouse down states ‘Flavours’ of a widget such as primary and secondary button styles. Toggleable elements within a widget such as buttons or bullets within a product card. How to turn a collection of components into a basic design system. Prototyping fundamentals: How to make journeys clickable. How to introduce animations. Tips and tricks for effective user testing. The limitations of Figma prototyping Is it for you? With no experience of Figma necessary, this course is suitable if: If you’re involved with creating or curating digital experiences. if you want to turn your design ideas into something tangible. If you work with Figma designers and want to understand the ‘art of the possible’. If you want to collaborate with your team on Figma projects. Learning objectives After completing day 1 you will be able to: Understand the Figma user interface. Create mobile and desktop interface designs. Effectively collaborate remotely. Share designs and gather feedback. Use auto layout to promote consistent and usable designs. Understand Figma’s role in user experience design, and its limitations. After day 2 you will be able to: Understand how design systems are set up and used. Create component variants for use in your own design system. Use that design system to efficiently create a user journey. Create a prototype suitable for user testing.

Addigy Certified Expert (ACE) Course
By Influential Training
Addigy ACE course, Addigy certificate, Addigy Training
