- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
133 Courses in Cardiff delivered Live Online
Red Hat Application Development I: Programming in Java EE (AD183)
By Nexus Human
Duration 5 Days 30 CPD hours This course is intended for This course is designed for Java developers who want to learn more about the specifications that comprise the world of Java Enterprise Edition (Java EE), Proficiency in developing Java SE applications, with 2+ years of experience required, Proficiency in using an IDE such as Red Hat Developer Studio or Eclipse, Experience with Maven is recommended but not required. Overview Red Hat Application Development I: Programming in Java EE Virtual Training exposes experienced Java Standard Edition (Java SE) developers to the world of Java Enterprise Edition (Java EE). As an experienced Java Standard Edition (Java SE) developer, this course introduces you to the world of Java Enterprise Edition (Java EE). You will learn about the various specifications that make up Java EE. Through hands-on labs, you will transform a simple Java SE command line application into a multi-tiered enterprise application using various Java EE specifications, including Enterprise Java Beans, Java Persistence API, Java Messaging Service, JAX-RS for REST services, Contexts and Dependency Injection (CDI), and JAAS for securing the application. This course is based on Red Hat© Enterprise Application Platform 7.0. 1 - TRANSITION TO MULTI-TIERED APPLICATIONS Describe Java EE features and distinguish between Java EE and Java SE applications. 2 - PACKAGE AND DEPLOY APPLICATIONS TO AN APPLICATION SERVER Describe the architecture of a Java EE application server, package an application, and deploy the application to an EAP server. 3 - CREATE ENTERPRISE JAVA BEANS Develop Enterprise Java Beans, including message-driven beans. 4 - MANAGE PERSISTENCE Create persistence entities with validations. 5 - MANAGE ENTITY RELATIONSHIPS Define and manage JPA entity relationships. 6 - CREATE REST SERVICES Create REST APIs using the JAX-RS specification. 7 - IMPLEMENT CONTEXTS AND DEPENDENCY INJECTION Describe typical use cases for using CDI and successfully implement it in an application. 8 - SECURE JAVA EE APPLICATIONS Use JAAS to secure a Java EE application. 9 - SECURE JAVA EE APPLICATIONS Use JAAS to secure a Java EE application. 10 - COMPREHENSIVE REVIEW OF RED HAT JBOSS DEVELOPMENT I: JAVA EE Demonstrate proficiency of the knowledge and skills obtained during the course. Additional course details: Nexus Humans Red Hat Application Development I: Programming in Java EE (AD183) training program is a workshop that presents an invigorating mix of sessions, lessons, and masterclasses meticulously crafted to propel your learning expedition forward. This immersive bootcamp-style experience boasts interactive lectures, hands-on labs, and collaborative hackathons, all strategically designed to fortify fundamental concepts. Guided by seasoned coaches, each session offers priceless insights and practical skills crucial for honing your expertise. Whether you're stepping into the realm of professional skills or a seasoned professional, this comprehensive course ensures you're equipped with the knowledge and prowess necessary for success. While we feel this is the best course for the Red Hat Application Development I: Programming in Java EE (AD183) course and one of our Top 10 we encourage you to read the course outline to make sure it is the right content for you. Additionally, private sessions, closed classes or dedicated events are available both live online and at our training centres in Dublin and London, as well as at your offices anywhere in the UK, Ireland or across EMEA.

Red Hat Application Development I: Programming in Java EE with exam (AD184)
By Nexus Human
Duration 5 Days 30 CPD hours This course is intended for This course is designed for Java developers who want to learn more about the specifications that comprise the world of Java Enterprise Edition (Java EE). Overview As a result of attending this course, you should be able to describe most of the specifications in Java EE 7 and create a component with each specification. You will be able to convert a Java SE program into a multi-tiered Java EE application. You should be able to demonstrate these skills: Describe the architecture of multi-tiered Java EE applications. Package Java EE applications and deploy to Red Hat JBoss Enterprise Application Platform with various tools. Create an Enterprise Java Bean instance. Manage the persistence of data using Java Persistence API. Create a web service using JAX-RS. Properly apply context scopes to beans and inject resources into Java Beans. Store and retrieve messages using the Java Messaging Service. Secure a Java EE application. Red Hat Application Development I: Programming in Java EE with exam (AD184) exposes experienced Java Standard Edition (Java SE) developers to the world of Java Enterprise Edition (Java EE). This course is based on Red Hat© Enterprise Application Platform 7.0. This course is a combination of Red Hat Application Development I: Programming in Java EE (AD183) and Red Hat Certified Enterprise Application Developer Exam (EX183). In this course, you will learn about the various specifications that make up Java EE. Through hands-on labs, you will transform a simple Java SE command line application into a multi-tiered enterprise application using various Java EE specifications, including Enterprise Java Beans, Java Persistence API, Java Messaging Service, JAX-RS for REST services, Contexts and Dependency Injection (CDI), and JAAS for securing the application. Transition to multi-tiered applications Describe Java EE features and distinguish between Java EE and Java SE applications. Package and deploying applications to an application server Describe the architecture of a Java EE application server, package an application, and deploy the application to an EAP server. Create Enterprise Java Beans Develop Enterprise Java Beans, including message-driven beans. Manage persistence Create persistence entities with validations. Manage entity relationships Define and manage JPA entity relationships. Create REST services Create REST APIs using the JAX-RS specification. Implement Contexts and Dependency Injection Describe typical use cases for using CDI and successfully implement it in an application. Create messaging applications with JMS Create messaging clients that send and receive messages using the JMS API. Secure Java EE applications Use JAAS to secure a Java EE application. Comprehensive review of Red Hat JBoss Development I: Java EE Demonstrate proficiency of the knowledge and skills obtained during the course. Additional course details: Nexus Humans Red Hat Application Development I: Programming in Java EE with exam (AD184) training program is a workshop that presents an invigorating mix of sessions, lessons, and masterclasses meticulously crafted to propel your learning expedition forward. This immersive bootcamp-style experience boasts interactive lectures, hands-on labs, and collaborative hackathons, all strategically designed to fortify fundamental concepts. Guided by seasoned coaches, each session offers priceless insights and practical skills crucial for honing your expertise. Whether you're stepping into the realm of professional skills or a seasoned professional, this comprehensive course ensures you're equipped with the knowledge and prowess necessary for success. While we feel this is the best course for the Red Hat Application Development I: Programming in Java EE with exam (AD184) course and one of our Top 10 we encourage you to read the course outline to make sure it is the right content for you. Additionally, private sessions, closed classes or dedicated events are available both live online and at our training centres in Dublin and London, as well as at your offices anywhere in the UK, Ireland or across EMEA.

BA28 - Requirements Analysis and Use Cases
By Nexus Human
Duration 2 Days 12 CPD hours This course is intended for This course has been expressly designed for the Business Analyst, Business System Analyst, System Analyst, and/or Requirements Engineer. The Designer, Developer and Tester who are actively involved in utilizing use cases may also benefit from this workshop. Overview Employ use cases to elicit requirements, at a business, system or subsystem levelEmploy use cases to document the scope of a projectWrite use cases in a clear and unambiguous way.Model use cases with workflow diagramsPlan and divide up the project work based on your use casesManage a use case as it evolves over time and goes through many changes This course provides a strong foundation in the mechanics of use case diagramming and writing textual descriptions of use cases. In this highly interactive workshop, you will learn how to enhance and refine your use case skills, how to involve your stakeholders in the use case process, and how to develop use cases that provide valuable information to the designers and testers. Use cases that meet the needs of designers can be too technical and too detailed for other stakeholders. Use cases that satisfy business users are usually not very helpful to designers and testers. How do you satisfy these two disparate interests? And how do you handle the details like business rules, data validations and user interface specifications? Introduction Course objectives Understanding of business analysis Knowledge areas of BABOK© Guide Overview of Use Cases Describe the purpose and value of a use case approach Use case terminology Introducing a use case approach Needs Assessment Purpose of Needs Assessment Needs Assessment and use cases Defining and understanding the strategic goals and objectives Understanding the business process Identifying actors Identifying business information Analyzing locations and operations Drawing the business use case diagram Building the business architecture Defining solution options Defining System Scope Product scope/project scope System actors versus business actors Analyzing the business process model Analyzing business use cases Identifying candidate use cases Identifying scenarios Diagramming use cases Draw a use case diagram Create a use case catalog Evaluating, Prioritizing, and Packaging Use Cases Iterative nature of this work Evaluating use cases Use case priority Use case risk Use case complexity Use case dependencies Evaluate and Prioritize use cases Dividing work up between releases Packaging A process for how to perform packaging Writing the Main Success Scenario Use case descriptions Primary and secondary goals Assumptions Pre-conditions Triggers Post-conditions Scenario example Main success scenario Conditional execution Use cases and requirements Best practices for writing a use case description Writing the Other Scenarios Scenarios and flows Alternate scenarios and flows Alternate vs. basic flow Guidelines for alternate flows Exception flows Failed post conditions Write alternate and exception flows Process Modeling to Describe Use Case Flows UML© Activity Diagram Notation Sequencing activities Developing an activity diagram Facilitated sessions Draw a UML© Activity Diagram Using Advanced Diagramming Techniques Commonality Dependency Relationships Identify relationships Comparing the different relationships Developing a Requirements Specification Requirements and use cases Detailed requirements Common approaches to specifications Non-functional requirements Write non-functional requirements User interface requirements UI data descriptions Business rules Decision tables and inference rules How to document simple calculations Reporting requirements Data requirements Data accessibility requirements Traceability Course Summary Wrap-up Finding more information Additional course details: Nexus Humans BA28 - Requirements Analysis and Use Cases training program is a workshop that presents an invigorating mix of sessions, lessons, and masterclasses meticulously crafted to propel your learning expedition forward. This immersive bootcamp-style experience boasts interactive lectures, hands-on labs, and collaborative hackathons, all strategically designed to fortify fundamental concepts. Guided by seasoned coaches, each session offers priceless insights and practical skills crucial for honing your expertise. Whether you're stepping into the realm of professional skills or a seasoned professional, this comprehensive course ensures you're equipped with the knowledge and prowess necessary for success. While we feel this is the best course for the BA28 - Requirements Analysis and Use Cases course and one of our Top 10 we encourage you to read the course outline to make sure it is the right content for you. Additionally, private sessions, closed classes or dedicated events are available both live online and at our training centres in Dublin and London, as well as at your offices anywhere in the UK, Ireland or across EMEA.

Online Options
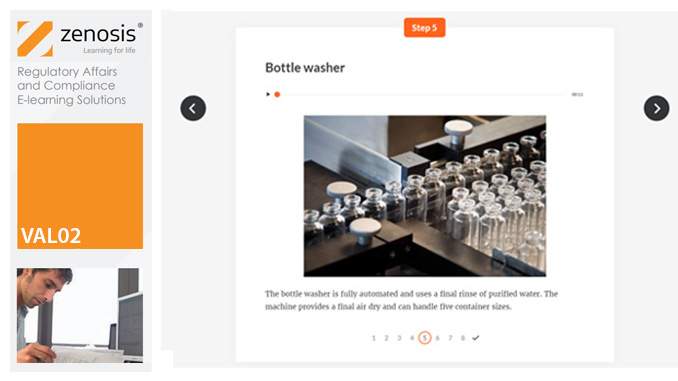
Show all 521VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
Pharmaceutical Validation Training Course (ONLINE) – For More Senior Validation, CQV and C&Q Roles
By Getreskilled (UK)
Take our part-time online “Conversion Course into Pharmaceutical Manufacturing“ NO Previous Industry Experience or Science Qualifications Required

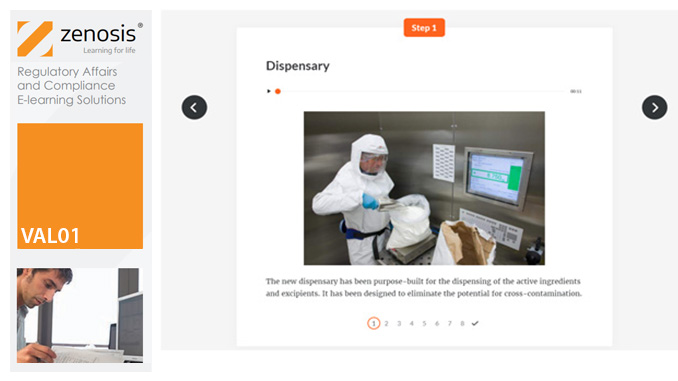
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
Equipment Validation Training Course (ONLINE) – For Starter Validation, CQV and C&Q Roles
By Getreskilled (UK)
Take our part-time online “Conversion Course into Pharmaceutical Manufacturing“ NO Previous Industry Experience or Science Qualifications Required

VAL05: Equipment Cleaning Validation
By Zenosis
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.
VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
Computer System Validation Training Course (ONLINE). Extend Your Role to CSV Projects. Get Certified and Become a CSV Professional
By Getreskilled (UK)
Has the Computer System Validation Engineer left and you’ve been handed their responsibilities? Do the thoughts of your next audit fill you with dread? CSV can be frustrating but this program will show you how to manage electronic data in a regulated manufacturing/laboratory/clinical environment using the GAMP framework and ensure compliance with FDA’s 21 CFR Part 11, EU Annex 11 or other regulatory guidelines.

VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

Professional Certificate Course in Requirements Validation and Management in London 2024
4.9(261)By Metropolitan School of Business & Management UK
This Professional Certificate Course in Requirements Validation and Management offers a thorough exploration of the importance of validating achievable and realistic requirements. Participants will delve into diverse validation techniques, including reviews, inspections, and walkthroughs, while also gaining insights into monitoring, tracking, and effectively managing conflicting requirements to meet stakeholder expectations throughout the project lifecycle. After the successful completion of the course, you will be able to learn about the following: Understand the importance of validating requirements to ensure that they are achievable and realistic Discuss different techniques for validating requirements, such as reviews, inspections, and walkthroughs Understand the importance of monitoring and tracking requirements throughout the project lifecycle Explain how to handle conflicting requirements and manage stakeholder expectations This Professional Certificate Course in Requirements Validation and Management provides a thorough grasp of validating achievable requirements using diverse techniques like reviews and inspections. Participants also learn to monitor, track, and address conflicting requirements, ensuring stakeholder satisfaction throughout the project lifecycle. This Professional Certificate Course in Requirements Validation and Management imparts crucial skills, emphasizing the significance of ensuring achievable and realistic requirements through diverse validation techniques, alongside monitoring, tracking, and effective conflict resolution for enhanced stakeholder satisfaction. Course Structure and Assessment Guidelines Watch this video to gain further insight. Navigating the MSBM Study Portal Watch this video to gain further insight. Interacting with Lectures/Learning Components Watch this video to gain further insight. Project Requirements Validation and Management Self-paced pre-recorded learning content on this topic. Requirements Validation and Management Put your knowledge to the test with this quiz. Read each question carefully and choose the response that you feel is correct. All MSBM courses are accredited by the relevant partners and awarding bodies. Please refer to MSBM accreditation in about us for more details. There are no strict entry requirements for this course. Work experience will be added advantage to understanding the content of the course.The certificate is designed to enhance the learner's knowledge in the field. This certificate is for everyone eager to know more and get updated on current ideas in their respective field. We recommend this certificate for the following audience, Project Managers Business Analysts Requirements Engineers Software Developers Stakeholders in Project Development Quality Assurance Professionals Average Completion Time 2 Weeks Accreditation 3 CPD Hours Level Advanced Start Time Anytime 100% Online Study online with ease. Unlimited Access 24/7 unlimited access with pre-recorded lectures. Low Fees Our fees are low and easy to pay online.


