- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Master Business Networking in Just 1 Day - Join our Workshop in Cardiff
By Mangates
Business Networking 1 Day Training in Cardiff

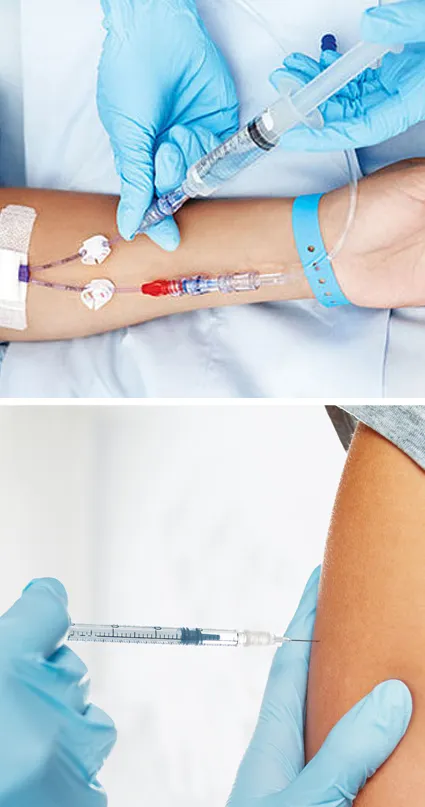
THIS COURSE PACKAGE INCLUDES: 1: PERIPHERAL I.V. CANNULATION - IV THERAPIES COURSE (GPT008) 2: VACCINATION / INJECTION COURSE (GPT601) Learn how to administer injectables and intravenous therapies ... FAST-TRACK YOUR AESTHETICS TRAINING WITH OUR COMPLETE TRAINING PACKAGE 20% Multi-Course Discount Cover all stages from Level 1 through to Level 4 (FDSc) Cover your theory training online Complete your advanced practical training in 1 day Practical training in Classroom or Virtual Classroom Comprehensive Practise@Home training kits for VC Awards 2 accredited qualifications Dual Accreditations for all courses Covers all steps required to safely perform injectables Covers all steps required to safely perform IV therapies Practise IV on artificial arm with fake blood Practise injection techniques on realistic injection pads Learn beginner to advanced skills and techniques Basic understanding of English language required OPEN TO ALL APPLICANTS
Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Exploration & Production (E&P) Accounting Level 3
By EnergyEdge - Training for a Sustainable Energy Future
Join EnergyEdge's classroom training course and gain valuable insights into Exploration and Production Accounting - Level 3. Enroll today!

FPSO Operations Management & Safety
By EnergyEdge - Training for a Sustainable Energy Future
About this Training Course This five-day training course will cover aspects of FPSO operations with an emphasis on management of safety. A background of the methodology used to generate the various safety plans and codes used in the operational safety case will be covered. The course will also review general operational guidelines in the offshore environment to ensure that the operations are completed as designed. This will include offtake operations and maintenance processes for the plant and vessel as well as a session on subsea. Training Objectives To enable participants to obtain an in-depth understanding of FPSO operations, safety and maintenance: Provide a broad overview of how an FPSO operation is set up and established in the field Grasp the various methods used to moor the production units Analyse information and methods required to establish the operation according to local and international regulations. Assess the methods used to calculate the safety parameters and requirements to construct an approved valid safety case Explore conditions to monitor during operations with regards to safety and structure Inspect various types of hazards associated with production and produced materials Review regular and emergency operational maintenance requirements Examine cyclones and weather events causing disconnection Comprehend manning, training requirements and operations including cargo Case studies and Exercises: The training course uses real life examples and case studies to explain the setup, preparation, implementation and operational activities required to successfully complete FPSO operations from a safety and maintenance perspective. This will allow the application of participants' newly-acquired knowledge. Case studies also stimulate independent thinking and discussion among the participants. Case Studies The case studies proposed for this course will include: A group exercise to consider what is the minimum requirement for the production of a safety case Generic plan for the development of a maintenance management system using computer software systems (e.g. Amos) Construction of a UWILD plan to include scope and potential tendering requirements Quizzes The various quizzes can be expanded or arranged to suit the group/interests and topics covered but will generally include the following topics: Mooring types and location where they are primarily used Safety cases Safety management systems Process hazards and mitigation UWILD components and regulations Operations management Offtake operations Target Audience The course is generally aimed at personnel who are involved in FPSO operations as either offshore crew or onshore support team members. This course will also greatly benefit the following groups but not limited to: Process engineers Surveyors Facility engineers Naval architects Operations engineer Health, Safety, Environment (HSE) specialists and managers Maintenance engineers Course Level Intermediate Training Methods The training course is presented in an interactive workshop format that allows for discussion. The course will be delivered through analysis of case studies and running examples of problems. Course Duration: 5 days in total (35 hours) The training instructor relies on a highly interactive training method to enhance the learning process. This method ensures that all participants gain a complete understanding of all the topics covered. The training comprises of information supplemented by visual activities including photographs from various operations and videos of operational and safety activities. Interaction verbally will be necessary to achieve the best learning outcome from the materials. Course timings and breaks 0830 - Registration 0900 - Start of training 1030 - Morning break 1045 - Training recommences 1230 - Lunch break 1330 - Training recommences 1515 - Afternoon break 1530 - Training recommences 1700 - End of training Trainer Your expert course leader has been involved in the Oil and Gas industry for over 28 years in several different roles commencing as a second deck officer on FPSO's and moving through the industry from deck officer to Master, OIM and Field Superintendent. He has also been in charge of semisubmersible and fixed production platforms as field superintendent before moving ashore into senior management roles. On completion of industry specific activities, he moved to teaching roles at South Tyneside Maritime College in the department of marine and simulation and presented live courses to maritime students from cadet to Master. Prior to returning to Perth in March 2019, he was training manager for a subsidiary of Gaz Transport and Technigaz of France. He has a Master Class 1 certificate with endorsements for hazardous cargoes and a Graduate Certificate in Business Administration as well as multiple course certificates required to operate FPSO's, platforms and semisubmersible facilities. He has been involved in the preparation of national standards for Safety Cases (Australia); Designing and structural setup of competency-based training systems in South East Asia. He was also involved in the development of minimum standards, competency profiles position based and preparation and implementation of training plans to ensure competency in the local staff in Asian operations. He was part of the development team for minimum standards of offshore safety in Australian offshore operations including the implementation of the Common Safety Training Program (CSTP). He was also involved in implementing training plans for both simulator and classroom based courses in LNG operations and cargo tank design and construction. Highlighted work experience: Premier Petroleum Myanmar Ltd Petronas Carigali Newfield Australia (Cartier) Pty Ltd: FPSO Jabiru Venture FPSO Challis Venture Onshore OIM Australian FPSO Management Pty. Ltd. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

Quality Assurance for Good Laboratory Practice
By Research Quality Association
Course Information A must-have programme for Quality Assurance auditors stepping into or honing their role within a Good Laboratory Practice (GLP) environment, this course offers invaluable, expert guidance for crafting a robust and efficient GLP audit programme. What will I learn? A solid regulatory foundation underpinning quality assurance activities Clarity on the roles of Quality Assurance, management, and study director within the framework of Good Laboratory Practice principles Enhanced efficacy in inspections and audits Heightened compliance with Good Laboratory Practice standards for your facility Unique insights into governmental monitoring activities within the GLP sphere. This course is structured to encourage delegates to Discuss and develop ideas Solve specific problems Examine particular aspects of GLP. Tutors Tutors will be comprised of (click the photos for biographies): Cate Ovington Director, The Knowlogy Group Ltd Jane Elliston Senior Quality Assurance Auditor, Battelle UK Shona Ross Head of QA, Tower Mains Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Welcome and Introductions 09:15 Good Laboratory Practice Standards and Regulations An insight into the background and history of Good Laboratory Practice. 09:45 Principles of Quality Assurance What is the role and responsibilities of QA in GLP. Maintaining the independence of QA and what is an audit. 10:30 Break 10:45 Standard Operating Procedures GLP requirements and QA involvement. 11:30 Study Plans GLP requirements and QA involvement. 12:05 QA Programme Risk based programme, what are study, process and facility audits. 13:00 Lunch 14:00 Inspections Attitudes, techniques and attributes. 14:40 Workshop 1 - Facility and Process Inspections An exercise in inspection planning and preparation for inspections. 15:15 Break 15:30 Workshop 1 - Feedback 15:45 The Auditor and Audit Conduct Attitudes, attributes and techniques. 16:30 Panel Session An opportunity for delegates to put questions to the panel of speakers. 17:15 Close of Day Day 2 09:00 Workshop 2 - A Mock Audit 10:45 Break 11:00 Workshop 2 - Feedback 11:30 Auditing the Study Report Techniques and methods for the QA audit of the study report. 12:00 Record Keeping and Data The impact of GLP on data and records management. 12:40 Lunch 13:25 Data Integrity A look at the OECD GLP guidance document; the expectations of the regulators and the involvement of QA - Where QA adds value. 14:15 Workshop 3 - Amendments to Study Plan and Deviations from the Plan What are they? What is the difference between them? How are they controlled? 15:00 Workshop 3 - Feedback 15:15 Break 15:30 Regulatory Compliance GLP Monitoring Authority monitoring for compliance with Good Laboratory Practice. 16:15 Panel Session An opportunity for delegates to put questions to the panel of speakers. 16:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Learn

Full-Time Course : Advanced Course in Bespoke Tailoring and Cutting
By Savile Row Bespoke Academy
The Savile Row Academy Advanced Course in Bespoke Tailoring and Cutting starts each September. The course is a modular programme of advanced study modules including; pattern drafting, cloth cutting, fitting and remarking; Waistcoat making; Trouser making; Coat making. Students will complete one three-piece suit and successful trainees will be awarded a Bespoke Tailor’s Certificate.

Practical Approach to Auditing Systems and Processes
By Research Quality Association
Course Information Our extensively proven course delves into the essential stages of process and system auditing. Gain invaluable insights and direction in auditing systems and processes, spanning across global and local organisational levels. This course will assist delegates with: A practical approach for the development and conduct of process and system audits An enhanced understanding of key system audit principles, preparation, design and conduct Increased expertise, efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Share knowledge and experiences. By the end of the course delegates will be better able to: Design and plan more effectively to achieve their process and systems audit objectives and add value to their organisation Improve the effectiveness, focus and credibility of the audit programme Understand the key system audit principles, preparation, design and conduct Develop system audit tools to ensure more effective audit conduct and outcome Create audit strategies utilising risk management principles Prepare for inspections. Tutors Tutors will be comprised of (click the photos for biographies): Allison Jack Executive Director, Bristol Myers Squibb Rocio Castellanos Director, Pfizer Ltd Guy Houben G(C)LP Auditor, Janssen Pharmaceutical Companies of Johnson & Johnson Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introductions, Expectations/Challenges/Experiences A discussion to explore the range of approaches to the conduct of systems audit. 09:30 Introducing Systems Audit What is a system? Why conduct system audits? Advantages, disadvantages and challenges. 10:20 Break 10:35 Systems Audit Design and Planning Identifying the customer, setting objectives, development of the audit plan and audit tools, plans for the audit report. 12:00 Designing System Audit Tools 12:45 Lunch 13:30 System Audit Plan - Exercise 14:00 Introduction to Case Studies The objectives of the case studies are defined and process and outputs described. 14:15 Case Studies - Session 1 A first opportunity for work on case studies. Defining objectives and scope and understanding the requirements of the audit client. 15:00 Break 15:20 Case Studies - Session 1 continued 16:30 Case Studies - Feedback 17:00 Close of Day 1 Day 2 09:00 Simple System Audit Example - Introduction The objectives of the case studies are defined and process and outputs described. 09:10 Case Studies - Session 2 - A Simple System Audit Example An example of system audit applied to a simple system. 10:30 Break 10:45 A Simple System Audit Example - Case Study Feedback 11:30 Strategy Audit programme planning. 12:15 Lunch 13:00 Case Studies - Session 3 Work on delegate's case studies. 14:30 Break 14:45 Case Studies - Session 3 - Feedback 15:15 Closing remarks 15:30 Close of course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

Master Business Networking in Just 1 Day - Join our Workshop in Carlisle
By Mangates
Business Networking 1 Day Training in Carlisle

Search By Location
- break, Courses in London
- break, Courses in Birmingham
- break, Courses in Glasgow
- break, Courses in Liverpool
- break, Courses in Bristol
- break, Courses in Manchester
- break, Courses in Sheffield
- break, Courses in Leeds
- break, Courses in Edinburgh
- break, Courses in Leicester
- break, Courses in Coventry
- break, Courses in Bradford
- break, Courses in Cardiff
- break, Courses in Belfast
- break, Courses in Nottingham
