- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
66 Courses
Applied E&P Economics – Commercial Decisions & Fiscal Systems
By EnergyEdge - Training for a Sustainable Energy Future
Enhance your knowledge in applied EP economics and commercial decisions with EnergyEdge course. Enroll now to secure a spot in our course.
Petroleum Risk Analysis & Portfolio Management
By EnergyEdge - Training for a Sustainable Energy Future
Elevate your knowledge in petroleum risk analysis and portfolio management with Energyedge. Sign up with us today!
Project Economics, Risk and Decision Analysis for Oil & Gas
By EnergyEdge - Training for a Sustainable Energy Future
EnergyEdge's classroom training provides in-depth knowledge on project economics, risk assessment, and decision analysis for the oil and gas industry. Take your skills to the next level.

Piping Stress Engineering
By EnergyEdge - Training for a Sustainable Energy Future
About this Virtual Instructor Led Training (VILT) The 5 half-day Piping Stress Engineering Virtual Instructor Led Training (VILT) course will systematically expose participants to: The theory and practice of piping stress engineering, with special reference to ASME B 31.1 and ASME B 31.3 Standards. The basic principles and theories of stress and strain and piping stress engineering, through a series of lessons, case study presentations, in-class examples, multiple-choice questions (MCQs) and mandatory exercises. Principal stresses and shear stresses which form the backbone of stress analysis of a material. Expressions for these quantities will be derived using vector algebra from fundamentals. Thermal stress-range, sustained and occasional stresses, code stress equations, allowable stresses, how to increase flexibility of a piping system, cold spring. The historical development of computational techniques from hand calculations in the 1950s to the present-day software. Training Objectives On completion of this VILT course, participants will be able to: Identify potential loads the piping systems and categorise the loads to primary and secondary. Determine stresses that develop in a pipe due to various types of loads and how to derive stress-load relationships, starting from scratch. Treat the primary and secondary stresses in piping system in line with the intent of ASME Standards B 31.1 and B 31.3 and understand how the two codes deal with flexibility of piping systems, concepts of self-springing and relaxation/shake down, displacement stress range and fatigue, what is meant by code compliance. Understand the principles of flexibility analysis, piping elements and their individual effects, flexibility factor, flexibility characteristic, bending of a curved beam and importance of virtual length of an elbow in the flexibility of a piping system. Learn stress intensification factors of bends, branch connections and flanges. Understand how the stresses in the material should be controlled for the safety of the piping system, the user and the environment. Examine how codes give guidance to determine allowable stresses, stress range reduction due to cyclic loading, and effects sustained loads have on fatigue life of piping. Confidently handle terminal forces and moments on equipment. Understand the supplementary engineering standards required to establish acceptance of the equipment terminal loads and what can be done when there are no engineering standard governing equipment terminal loads is available and learn the techniques of local stress analysis. Get a thorough understanding of the concepts and the rules established by the ASME B 31.1 and ASME B 31.3 Standards. Perform flange load analysis calculations based on Kellogg's Equivalent Pressure method & Nuclear Code method. Perform the same using a piping stress analysis software and check for flange stresses and leakage. Confidently undertake formal training of piping stress analysis using any commercial software, with a clear understanding of what happens within the software rather than a 'blind' software training and start the journey of becoming a specialist piping stress engineer. Target Audience The VILT course is intended for: Recent mechanical engineering graduates who desire to get into the specialist discipline of Piping Stress Engineering. Junior mechanical, chemical, structural and project engineers in the industry who wish to understand the basics of Piping Stress Engineering. Engineers with some process plant experience who desire to progress into the much sought-after specialist disciplines of Piping Stress Engineering. Mechanical, process and structural engineers with some process plant experience who desire to upskill themselves with the knowledge in piping stress engineering and to become a Piping Stress Engineer. Any piping engineer with some pipe stressing experience in the industry who wish to understand the theory and practice of Piping Stress Engineering at a greater depth. A comprehensive set of course notes, practice exercises and multiple-choice questions (MCQs) are included. Participants will be given time to raise questions and participants will be assessed and graded based on responses to MCQs and mandatory exercises. A certificate will be issued to each participant and it will carry one of the three performance levels: Commendable, Merit or Satisfactory, depending on how the participant has performed in MCQs and mandatory exercises. Training Methods The VILT course will be delivered online in 5 half-day sessions comprising 4 hours per day, with 2 breaks of 10 minutes per day. Course Duration: 5 half-day sessions, 4 hours per session (20 hours in total). Trainer Your expert course leader is a fully qualified Chartered Professional Engineer with over 40 years of professional experience in Oil & Gas (onshore and offshore), Petrochemical and Mining industries in engineering, engineering/design management and quality technical management related to plant design and construction. At present, he is assisting a few Perth based oil & gas and mining companies in detail engineering, piping stress analysis, feasibility study and business development work related to plant design. He is a pioneer in piping stress engineering in Western Australia. His recent major accomplishments include the following roles and challenges: Quality Technical Support Manager of USD 54 billion (Gorgon LNG Project). This encompassed management of quality technical services connected with Welding, Welding Related Metallurgy, Non-Destructive Examination, Insulation /Refractory /Coating, AS2885 Pipelines Regulatory Compliance and Pressure Vessel Registration. Regional Piping Practice Lead and Lead Piping Engineer of Hatch Associates. In this role, he was responsible for providing discipline leadership to several mining projects for BHP Billiton (Ravensthorpe), ALCOA-Australia (Alumina), Maáden Saudi Arabia (Alumina), QSLIC China (Magnesium), COOEC China (O&G Gorgon). He was actively involved in the development of piping engineering practice in WA, including training and professional development of graduate, junior and senior engineers. This also includes the formation of the Piping Engineering Specialist Group. Lead Piping/Pipe Stress Engineer on ConocoPhillips' (COP) Bayu Undan Gas Recycle, Condensate production and processing platform. He was able to develop several novel design methodologies for the project and provided training to engineers on how to implement them. These methodologies were commended by COP and the underwriters of the project Lloyds Register of Shipping, UK. Creator of Piping Engineering Professional Course aimed at global engineering community. Professional Affiliations: Fellow, Institution of Mechanical Engineers, UK (IMechE) Fellow, Institution of Engineers, Australia (EA), National Register of Engineers (NER) Member American Society of Mechanical Engineers, USA (ASME) Honorary Life Member, Institution of Engineers, Sri Lanka (IESL) POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information about post training coaching support and fees applicable for this. Accreditions And Affliations

Online & interactive Women's Health and Holistic wellbeing course.
By lindsay wild
This is an accredited course with the International practitioners of Holistic medicine for women personally or professionally to transform their life. Relax, be calm, you've got this!

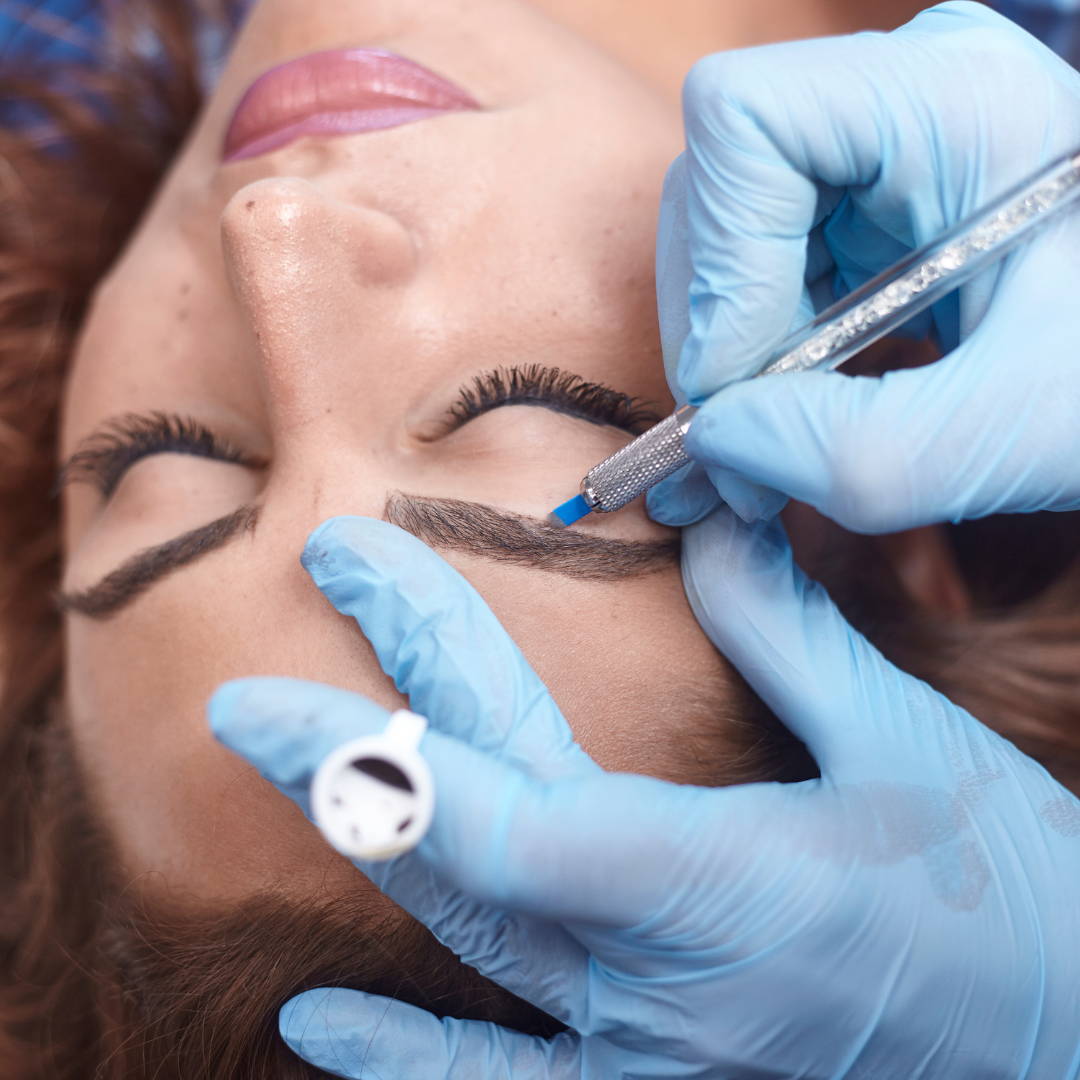
VTCT Level 4 Certificate in Enhancing Eyebrows with Microblading Techniques
By Cosmetic College
The VTCT Level 4 Certificate in Enhancing Eyebrows with Microblading Techniques is an advanced therapies qualification aimed at 18+ learners, who wish to pursue a career as a microblading technician. This qualification is based on attaining professional competence within the discipline of microblading and includes the required elements to work effectively as a microblading technician. Learners must achieve all mandatory units: Health and safety in the salon Client care and consultation Enhance appearance using microblading techniques Anatomy and physiology for microblading techniques Eyebrow shaping services Throughout this qualification, learners will develop their knowledge and understanding of the process of microblading. They will also develop the ability to apply practically the knowledge, understanding and skills to enable them to enhance eyebrow with microblading techniques. In parallel, learners will develop their communication skills, consultation and client care skills and time management skills, all of which are valued highly by employers. Learners are required to complete a series of assessment for each unit, verifying the learner's understanding and practical implementation of the knowledge and skills required to attain professional competence. Within the microblading unit, learners are required to produce evidence of a series of case studies, consolidating and demonstrating consistent applications of all the knowledge and practical skills in one place.
VTCT Level 4 Certificate in Enhancing Eyebrows with Microblading Techniques
By Cosmetic College
The VTCT Level 4 Certificate in Enhancing Eyebrows with Microblading Techniques is an advanced therapies qualification aimed at 18+ learners, who wish to pursue a career as a microblading technician. This qualification is based on attaining professional competence within the discipline of microblading and includes the required elements to work effectively as a microblading technician. Learners must achieve all mandatory units: Health and safety in the salon Client care and consultation Enhance appearance using microblading techniques Anatomy and physiology for microblading techniques Eyebrow shaping services Throughout this qualification, learners will develop their knowledge and understanding of the process of microblading. They will also develop the ability to apply practically the knowledge, understanding and skills to enable them to enhance eyebrow with microblading techniques. In parallel, learners will develop their communication skills, consultation and client care skills and time management skills, all of which are valued highly by employers. Learners are required to complete a series of assessment for each unit, verifying the learner's understanding and practical implementation of the knowledge and skills required to attain professional competence. Within the microblading unit, learners are required to produce evidence of a series of case studies, consolidating and demonstrating consistent applications of all the knowledge and practical skills in one place.

A System Approach to Good Pharmacovigilance Practice
By Research Quality Association
Course Information In today's evolving landscape, pharmacovigilance (PV) systems face ongoing challenges due to global, national, and company-specific events. This course focuses on developing personnel equipped to navigate these complexities and improve the safe use of medicinal products. We emphasise continuous global thinking, communication, and strategic planning, ensuring adaptability across various levels. The course explores maintaining PV system functionality while adhering to regulatory requirements. Participants will apply European regulatory standards to enhance and sustain PV system effectiveness, contributing to improvement initiatives and ensuring operational integrity. The course covers PV system intricacies, regulatory compliance, and a 'systems approach' for auditors, quality assurance personnel, and PV practitioners. Through presentations and workshops, attendees gain insights into implementing and maintaining an effective PV system. Who should attend? Auditors Pharmacovigilance Quality System Managers Pharmacovigilance scientists The QPPV. Course benefits Throughout the course delegates will explore application of the legal requirements for the PV system and quality system and how to assure these systems. They will demonstrate their ability to contribute to: A systematic investigation of the pharmacovigilance system and its quality system Examination of how the pharmacovigilance system and quality system interact to achieve compliance. The risk-based approach to auditing the PV system and quality system The maintenance of 'inspection readiness' Explore how to investigate the complex PV system Discussions about how to monitor and maintain the PV system and assure compliance. Course Objectives Clarify what has to be done: Explore application of the legal requirements. Explore how to do what has to be done: Adopt a systemic approach to systematically investigate or implement and maintain the PV system and quality system Examine how a compliant PV system and a compliant quality system interact to achieve compliance with regulatory requirements for PV Explore how to investigate the complexity of the PV system. Discus how to identify what is missing or what needs to be improved: Discuss how to monitor and maintain the PV system and assure compliance. This course will assist delegates with: An understanding of key system principles, A practical approach to implementing, maintaining and monitoring the PV system and its quality system A procedure to share expertise to increase efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas - Share knowledge and experiences - Solve specific problems. By the end of the course delegates will be able to: Understand better the pharmacovigilance system, its quality system and how the components interact to achieve the objectives of pharmacovigilance Investigate, and analyse the pharmacovigilance system and to identify what is missing and what needs to be improved. Tutors Tutors will be comprised of (click the photos for biographies): Jana Hyankova Head of PV Department, IVIGEE Services a.s. Programme Please note timings may be subject to alteration. Day 1 08:30 Welcome, registration, course objectives and introduction to work groups Housekeeping notices, meet other delegates, explore how to work in your work group, course objectives. Clarify the definition and objectives of Pharmacovigilance. 09:30 The Regulatory Framework for Pharmacovigilance Identify the relevant regulations and directives. Explore GVP guidance, structure of the modules and standard format of each module. 10:00 The Pharmacovigilance System Exploration of how to organise what has to be done, communications. Exploration of the structures and processes for pharmacovigilance. 10:30 Break 11:00 Workshop 1 and Feedback Exploring an organisational model of the pharmaceutical company- cooperation between PV and other stakeholders. 12:00 The Quality System for pharmacovigilance Exploration of the structures, processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 13:00 Lunch 14:00 Workshop 2 and Feedback The quality system puzzle. Explore the organisation of the PV quality system and how it interacts with the PV system. 14:30 The Quality System for pharmacovigilance Exploration of the structures processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 15:00 Description of PV System 15:30 Break 15:30 Workshop 3 and Feedback The quality system puzzle Explore the organisation of the PV quality system and how it interacts with the PV system. 16:00 The Pharmacovigilance Safety Master File Construction of the Pharmacovigilance System Master File and its purpose. 17:00 Workshop 3 and Feedback Description of PV System. 18:00 End of Day Day 2 08:30 Drug Safety in the Clinical Trial Environment - Part 1 Information flow and responsibilities of the sponsor. 09:30 Workshop 4 and Feedback Drug Safety in the Clinical Trial environment: Information flow and responsibilities of the sponsor. 10:30 Break 11:00 Drug Safety in the Clinical Trial Environment - Part 2 Information flow and responsibilities of the sponsor. 12:00 Lunch 13:00 Workshop 5 and Feedback Drug Safety in the clinical trial environment: Information flow and responsibilities of the sponsor. 13:30 Processing of Safety Data Exploration of safety data processing, verification, validation, follow up, formatting and collation, reporting requirements, quality and data management. 15:00 Break 15:30 EudraVigilance Exploration of how EudraVigilance supports the PV system. 16:15 Signal Detection and Evaluation/Risk Benefit Assessment: Pharmacovigilance Risk Assessment Committee (PRAC): What is a signal? What are the regulatory requirements? How is signal detection and evaluation conducted? Qualitative and quantitative methods of signal detection. Risk benefit assessment. 17:00 Risk Management Plans A cornerstone of Pharmacovigilance safety communications, direct healthcare professional communication 18:00 End of Day Day 3 08:30 The Pharmacovigilance Risk Assessment Committee (PRAC) Exploration of how good practice is achieved. Composition, role and responsibilities. Examples of referrals. 09:15 Development Safety Update Reports (DSURs): Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:30 Break 11:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 12:00 Workshop 6 and Feedback To explore the compilation and submission of the PSUR. 13:00 Lunch 13:30 Role of the QPPV Exploration of the legal responsibilities of the QPPV and the MAH. 14:30 Break 15:00 Workshop 7 and Feedback To explore the challenges faced by the QPPV. 15:30 End of course Extra Information Face-to-Face Course Course material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 23 Points Development Level Develop

Accredited Diploma in Aromatherapy Massage (Private 121)
By Renew Therapies Wellbeing Centre & Training Acdemy
Aromatherapy massage is a popular mainstream therapy, and is offered at any reputable spa or beauty treatment clinic. Our comprehensive 2-day professional course guides you carefully through everything you need to know to carry out an aromatherapy treatment for your clients. You will learn about the properties of essential oils, blending essential oils to suit your clients needs, also carrier oils and their own properties.

PgMP Exam Prep
By IIL Europe Ltd
PgMP® Exam Prep This course is designed and developed by PgMP® certified consultants and instructors. Its aim is to prepare professionals who are familiar with the principles of program management for the Program Management Professional (PgMP)® Examination. The course is based on PMI's The Standard for Program Management, A Guide to the Project Management Body of Knowledge (PMBOK® Guide), and PMI's Program Management Professional (PgMP)® Examination Content Outline (current versions). Through this learning experience, you will explore: Program management from a PMI standard perspective, including the interdependencies between the five performance domains, the three program phases, and the ten supporting activities in this new and improved program management standard The difference between the five performance domains in the new program management standard and the five practice domains in the examination content outline The role and competencies of the program manager The difference between project managers and program managers - and their relationship in a program environment The difference between program managers and portfolio managers - and their relationship in a program environment How program managers align and manage benefits The best ways to engage and involve program stakeholder groups How to establish governance across the program life cycle What You Will Learn At the end of this course, you will be able to: Differentiate between the practice domains in the PMI PgMP® Examination Content Outline and the performance domains in The Standard for Program Management - Fourth Edition Name and describe the three phases in the program management life-cycle phases Describe the mapping of the life-cycle phases with the supporting program activities Identify the key outputs of the supporting program activities Articulate the interrelationships between the program management supporting processes and the mapping of processes to Knowledge Areas and Process Groups in the PMBOK® Guide - Sixth Edition Apply program management knowledge to answer foundation and scenario-based questions Summarize the process and eligibility criteria for earning the PgMP® credential Getting Started Introductions Course structure Course goals and objectives Foundation Concepts Programs, projects, and portfolio definitions differences, and how they relate The definition of a component and how it relates to a program Representative program management life cycle Role of the program manager and the program office The difference between the program management practice and performance domains Program Register and Knowledge Asset Management Program registers, and how they are used to manage knowledge assets Knowledge asset management, beginning with the data, information, knowledge, and wisdom (DIKW) Model Knowledge assets and relationship to the performance domains The program manager as a knowledge asset manager Types of Programs Perspectives on programs to establish the 'right' perspective Categories of programs based on the program standard Scenario-based questions Program and Organization Strategy Alignment An overview of the Program Strategy Alignment performance domain Exploration of the elements of strategic alignment, i.e., the business case, program charter, and program roadmap Exploration of organization maturity and strategic alignment Scenario-based questions that reference both the Program Strategy Alignment performance domain and the Strategic Program Management practice domain Program Benefits An overview of the Program Benefits Management performance domain Exploration of each benefits management interaction with the representative program management life cycle: Benefits IdentificationBenefits Analysis and PlanningBenefits DeliveryBenefits TransitionBenefits SustainmentScenario-based questions that reference both the Program BenefitsManagement performance domain and the Benefits Management practice domain Program Stakeholder Engagement An overview of the Program Stakeholder Engagement performance domain Exploration of each stakeholder engagement performance domain activity: Program Stakeholder IdentificationProgram Stakeholder AnalysisProgram Stakeholder Engagement PlanningProgram Stakeholder EngagementProgram Stakeholder CommunicationsScenario-based questions that reference both the Program StakeholderEngagement performance domain and the Stakeholder Management practice domain Program Governance An overview of the Program Governance performance domain Exploration of each program governance performance domain activity: Program governance practicesProgram governance roles and responsibilitiesProgram governance design and implementationGovernance relationship within programsScenario-based questions that reference both the Program Governance performance domain and the Governance practice domain Program Life Cycle Management An overview of the Program Life Cycle Management performance domain Exploration of the three phases in the representative program life cycle: Program DefinitionProgram DeliveryProgram ClosureExploration of the interaction between program activities and integration managementScenario-based questions that reference both the Program Life CycleManagement performance domain and the Program Life Cycle practice domain Program Management Supporting Activities - Part 1 An overview of the program management supporting activities Exploration of 5 of 10 supporting activities: Program change managementProgram communications managementProgram financial managementProgram information managementProgram procurement managementScenario-based question(s) presented after each supporting activity Program Management Supporting Activities - Part 2 Exploration of the remaining 6 of 10 supporting activities: Program quality managementProgram resource managementProgram risk managementProgram schedule managementProgram scope managementScenario-based question(s) presented after each supporting activity Program Management Professional (PgMP®) Examination Application process and timeline General and special eligibility criteria International Institute's Online Learning Tool - access to sample examination questions Program Management Professional (PgMP®) Examination breakdown of domains and subdomains Terms and conditions of the exam PgMP® Professional Code of Conduct

Search By Location
- professional coaching Courses in London
- professional coaching Courses in Birmingham
- professional coaching Courses in Glasgow
- professional coaching Courses in Liverpool
- professional coaching Courses in Bristol
- professional coaching Courses in Manchester
- professional coaching Courses in Sheffield
- professional coaching Courses in Leeds
- professional coaching Courses in Edinburgh
- professional coaching Courses in Leicester
- professional coaching Courses in Coventry
- professional coaching Courses in Bradford
- professional coaching Courses in Cardiff
- professional coaching Courses in Belfast
- professional coaching Courses in Nottingham