- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
35 Courses
Level 7 NVQ Diploma in Construction Senior Management
By Dynamic Training and Assessments Ltd
Level 7 NVQ Diploma in Construction Senior Management

Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

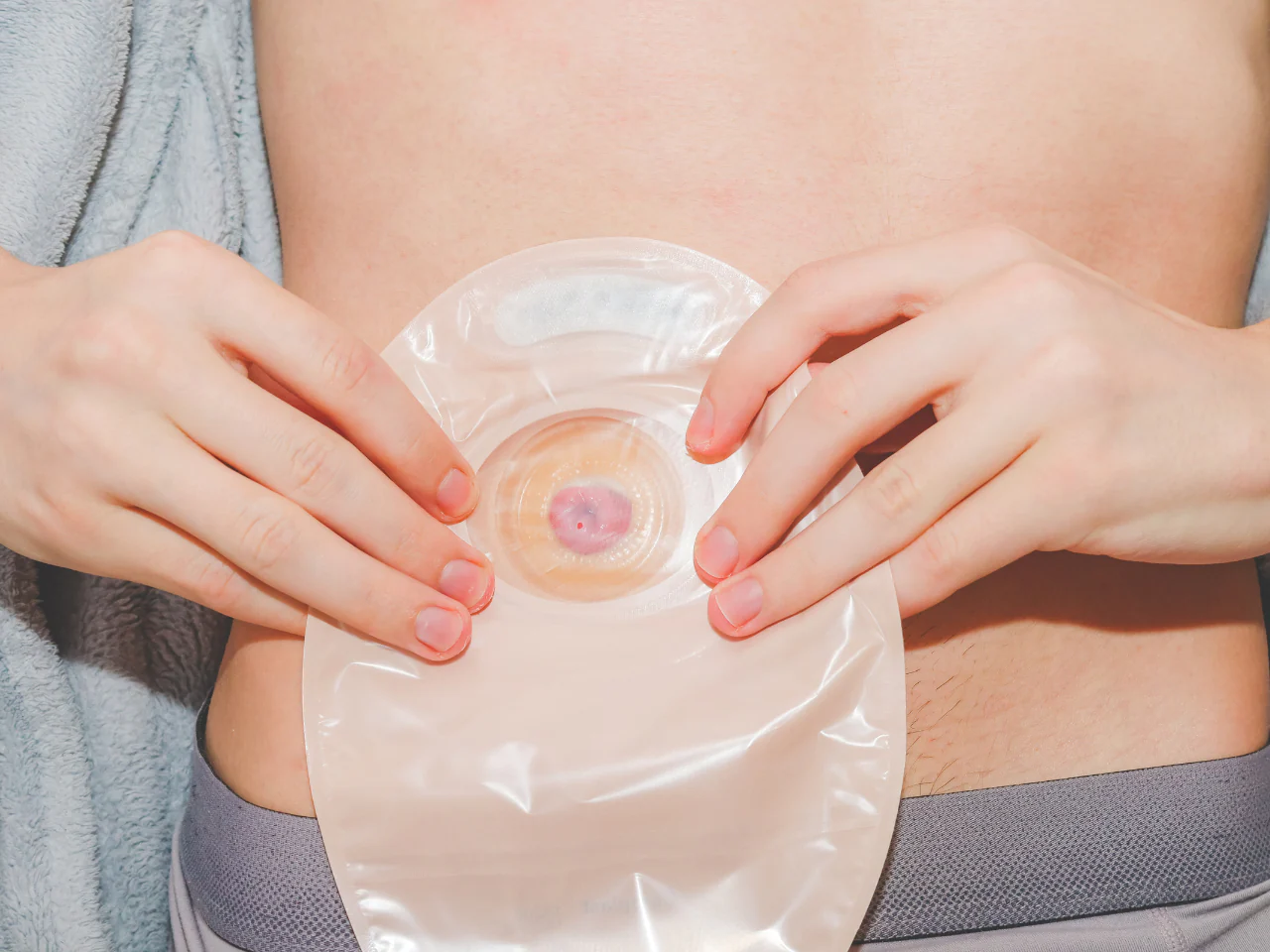
An Understanding of Stoma Care
By Guardian Angels Training
Gain essential knowledge and practical skills to provide effective care for patients with stomas with our "An Understanding of Stoma Care" course. Learn how to maintain stoma health and support patients through their journey.
Absence Management
By Inovra Group
Overview This course has been created to help safely navigate attendees through the minefield of absence management, paying attention to issues of systems, procedures and organisational culture along the way. Using a selection of exercises, activities and sample documents, the course examines some traditional methods of management as well as some more contemporary and innovative ways of keeping a lid on casual absence. Attendees will take away a number of practical tools and ideas to enable them to target performance improvement when back at their desks. Description It’s estimated that absence from work costs the UK economy over £13 billion per year, with the ‘average’ employee taking around seven days off sick annually. The need for managers, HR people and leaders to control absenteeism is critical if a company is to survive and prosper. But just what is ‘absence’? And how do we go about managing it and reducing it wherever we can, without falling foul of employment law? As well as the usual training material, attendees on this course also receive several useful handouts and exercises relating to absence management. Topics covered: An Absence Management Model – this section identifies a simple model for managers to apply when dealing with absenteeism Defining Absence – the text book definition will help learners clearly understand what is meant by absence Types of Absence – unravelling the different types of absence and distinguishing between absence and leave Classifying Absence – by classifying types of absence, the learner can begin to get a steer on how to manage it Statistics – identifying the real cost of absence and looking at regional and sector differences Reasons for Absence – considering the high-level issues that have an impact on absence, like culture and job design Causes of Sickness – here the national league tables of sickness causes are discussed, giving the learner the chance to reflect on their own team or company Absence Management – Stage 1 – contracts, policies, procedures and legal entitlements are all examined here, to allow learners to get a grasp of what they have to know to Absence Management – Stage 2 – record keeping, costing absence and benchmarking provide the chance for analysis and understanding in the context of the learner’s own organisation Absence Management – Stage 3 – setting out the skills and interventions that managers need to apply in the effective management of absence, including: communication, professional advice, workplace issues and return to work interviews Traditional Approaches – an examination of performance management, sick pay, discipline, recruitment and selection and how these can lend themselves to effective absence management Reducing Absenteeism – through less traditional approaches, looking at ‘carrots and sticks’, targets, employee assistance, unusual initiatives and organisational culture. Recent Developments – considering the impact of ‘fit notes’ and potential pandemics. Who should attend This course has been designed for anyone that deals with absence or needs to have an awareness of the absence management process. This could include; Team leaders, supervisors, managers, HR professionals and anyone else involved in the management of people or organisations. Requirements for Attendance None.

An Understanding of Dysphagia and Safe Swallowing
By Guardian Angels Training
Gain the knowledge and skills to assess, manage, and support individuals with dysphagia through our comprehensive "Understanding Dysphagia and Safe Swallowing" course. Learn about its causes, assessment methods, and strategies for safe swallowing.

Intra-Aortic Balloon Pump
By M&K Update Ltd
An opportunity to develop essential knowledge and skills that are necessary for the safe care and management of a patient requiring an Intra-Aortic Balloon Pump (IABP)

EA/PA Excellence Workshop Live from London on the 25th of April 2024. Featuring Keynote speaker Reggie Love, former Special Assistant to 44th President of the USA, Barrack Obama. Panel featuring Sophie Chapman, assistant to Steven Bartlett entrepreneur, author, host of the diary of a CEO and youngest ever dragon on BBC's Dragon's Den, Victoria Wratten, CEO of the Executive & Personal Assistants Association. The workshop contains, panel talk, keynote talk and facilitation over key topics from Kate Wood over the course of the day.

Microsoft Access Advanced (now with live online classes)
By Microsoft Office Training
Course Objectives At the end of this course you will be able to: Do advance Table design Do advance Query design and Action Querys Do advance Form design with the use of macros and buttons Export and import data to and from different sources. 1 year email support service Take a look at the consistent excellent feedback from our corporate clients visiting our site ms-officetraining co uk With more than 20 years experience, we deliver courses on all levels of the Desktop version of Microsoft Office and Office 365; ranging from Beginner, Intermediate, Advanced to the VBA level. Our trainers are Microsoft certified professionals with a proven track record with several years experience in delivering public, one to one, tailored and bespoke courses. Tailored in company training courses: You can choose to run the course exactly as they are outlined by us or we can customise it so that it meets your specific needs. A tailored or bespoke course will follow the standard outline but may be adapted to your specific organisational needs. Advanced Table Design Advanced Field Properties Table Properties Advanced Query Design Advanced Naming Conventions Join Tables in Queries Manage Query Joins Use Self-Joins in Queries Summarise Data in Queries Parameter Queries Action Queries Crosstab Queries Advanced Form Design Create Subforms and Linked Forms Form Controls Command Buttons Form Properties Interface, Start-Up and Navigations Forms Working with Macros Create Single Macros Run Macros Work with Sub Macros Use Conditional Macros Run Macros from Buttons Assign Macros to Events Extending Data Reach Import Data Export Data Work with Linked Tables Managing Databases Object Dependencies Database Documenter Performance Analyzers Regular Management of a Database Access Database Security Who is this course for? Who is this course for? The course is aimed at all users who would like to obtain the necessary skills to create advanced table, query, form and reports as well as to automate tasks with the use of macros. Career path Career path Microsoft Office know-how can instantly increase your job prospects as well as your salary. 80 percent of job openings require spreadsheet and word-processing software skills

Microsoft Access Advanced - In-company / Bespoke
By Microsoft Office Training
Course Objectives At the end of this course you will be able to: Do advance Table design Do advance Query design and Action Querys Do advance Form design with the use of macros and buttons Export and import data to and from different sources. 1 year email support service Take a look at the consistent excellent feedback from our corporate clients visiting our site ms-officetraining co uk With more than 20 years experience, we deliver courses on all levels of the Desktop version of Microsoft Office and Office 365; ranging from Beginner, Intermediate, Advanced to the VBA level. Our trainers are Microsoft certified professionals with a proven track record with several years experience in delivering public, one to one, tailored and bespoke courses. Our competitive rates start from £550.00 per day of training Tailored training courses: You can choose to run the course exactly as they are outlined by us or we can customise it so that it meets your specific needs. A tailored or bespoke course will follow the standard outline but may be adapted to your specific organisational needs. Advanced Table Design Advanced Field Properties Table Properties Advanced Query Design Advanced Naming Conventions Join Tables in Queries Manage Query Joins Use Self-Joins in Queries Summarise Data in Queries Parameter Queries Action Queries Crosstab Queries Advanced Form Design Create Subforms and Linked Forms Form Controls Command Buttons Form Properties Interface, Start-Up and Navigations Forms Working with Macros Create Single Macros Run Macros Work with Sub Macros Use Conditional Macros Run Macros from Buttons Assign Macros to Events Extending Data Reach Import Data Export Data Work with Linked Tables Managing Databases Object Dependencies Database Documenter Performance Analyzers Regular Management of a Database Access Database Security Who is this course for? Who is this course for? The course is aimed at all users who would like to obtain the necessary skills to create advanced table, query, form and reports as well as to automate tasks with the use of macros. Career path Career path Microsoft Office know-how can instantly increase your job prospects as well as your salary. 80 percent of job openings require spreadsheet and word-processing software skills

Search By Location
- Management of Asthma Courses in London
- Management of Asthma Courses in Birmingham
- Management of Asthma Courses in Glasgow
- Management of Asthma Courses in Liverpool
- Management of Asthma Courses in Bristol
- Management of Asthma Courses in Manchester
- Management of Asthma Courses in Sheffield
- Management of Asthma Courses in Leeds
- Management of Asthma Courses in Edinburgh
- Management of Asthma Courses in Leicester
- Management of Asthma Courses in Coventry
- Management of Asthma Courses in Bradford
- Management of Asthma Courses in Cardiff
- Management of Asthma Courses in Belfast
- Management of Asthma Courses in Nottingham
