- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
186 Courses
Absence Management
By Inovra Group
Overview This course has been created to help safely navigate attendees through the minefield of absence management, paying attention to issues of systems, procedures and organisational culture along the way. Using a selection of exercises, activities and sample documents, the course examines some traditional methods of management as well as some more contemporary and innovative ways of keeping a lid on casual absence. Attendees will take away a number of practical tools and ideas to enable them to target performance improvement when back at their desks. Description It’s estimated that absence from work costs the UK economy over £13 billion per year, with the ‘average’ employee taking around seven days off sick annually. The need for managers, HR people and leaders to control absenteeism is critical if a company is to survive and prosper. But just what is ‘absence’? And how do we go about managing it and reducing it wherever we can, without falling foul of employment law? As well as the usual training material, attendees on this course also receive several useful handouts and exercises relating to absence management. Topics covered: An Absence Management Model – this section identifies a simple model for managers to apply when dealing with absenteeism Defining Absence – the text book definition will help learners clearly understand what is meant by absence Types of Absence – unravelling the different types of absence and distinguishing between absence and leave Classifying Absence – by classifying types of absence, the learner can begin to get a steer on how to manage it Statistics – identifying the real cost of absence and looking at regional and sector differences Reasons for Absence – considering the high-level issues that have an impact on absence, like culture and job design Causes of Sickness – here the national league tables of sickness causes are discussed, giving the learner the chance to reflect on their own team or company Absence Management – Stage 1 – contracts, policies, procedures and legal entitlements are all examined here, to allow learners to get a grasp of what they have to know to Absence Management – Stage 2 – record keeping, costing absence and benchmarking provide the chance for analysis and understanding in the context of the learner’s own organisation Absence Management – Stage 3 – setting out the skills and interventions that managers need to apply in the effective management of absence, including: communication, professional advice, workplace issues and return to work interviews Traditional Approaches – an examination of performance management, sick pay, discipline, recruitment and selection and how these can lend themselves to effective absence management Reducing Absenteeism – through less traditional approaches, looking at ‘carrots and sticks’, targets, employee assistance, unusual initiatives and organisational culture. Recent Developments – considering the impact of ‘fit notes’ and potential pandemics. Who should attend This course has been designed for anyone that deals with absence or needs to have an awareness of the absence management process. This could include; Team leaders, supervisors, managers, HR professionals and anyone else involved in the management of people or organisations. Requirements for Attendance None.

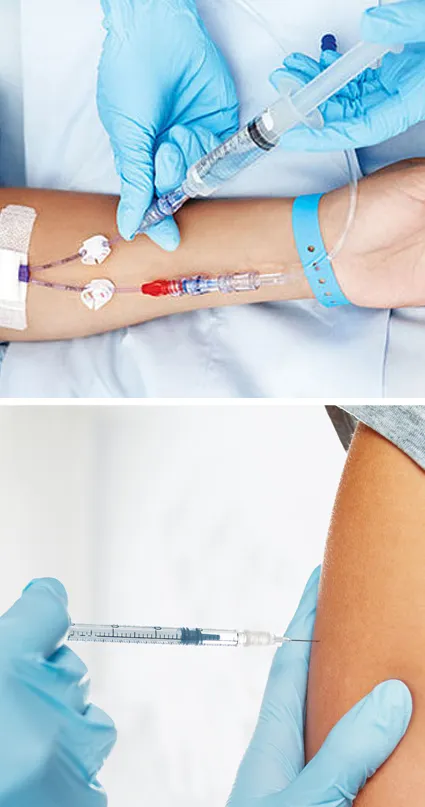
THIS COURSE PACKAGE INCLUDES: 1: PERIPHERAL I.V. CANNULATION - IV THERAPIES COURSE (GPT008) 2: VACCINATION / INJECTION COURSE (GPT601) Learn how to administer injectables and intravenous therapies ... FAST-TRACK YOUR AESTHETICS TRAINING WITH OUR COMPLETE TRAINING PACKAGE 20% Multi-Course Discount Cover all stages from Level 1 through to Level 4 (FDSc) Cover your theory training online Complete your advanced practical training in 1 day Practical training in Classroom or Virtual Classroom Comprehensive Practise@Home training kits for VC Awards 2 accredited qualifications Dual Accreditations for all courses Covers all steps required to safely perform injectables Covers all steps required to safely perform IV therapies Practise IV on artificial arm with fake blood Practise injection techniques on realistic injection pads Learn beginner to advanced skills and techniques Basic understanding of English language required OPEN TO ALL APPLICANTS
BOHS P903 - Management and control of evaporative cooling and other high risk industrial systems Online
By Airborne Environmental Consultants Ltd
BOHS P903 - Management and control of evaporative cooling and other high risk industrial systems is there to provide background and an overview of the risk of Legionella infection and how it can be controlled in Evaporative Cooling and other high risk Industrial type systems. It is a requirement of this course that candidates have successfully completed P901 - Legionella- Management and Control of Building Hot and Cold Water Services. Where both P901 and P903 courses are run on subsequent days or as a combined course then this prerequisite is waived.

BOHS P904 - Management and control in leisure, display, therapy and other non-industrial systems Online
By Airborne Environmental Consultants Ltd
BOHS P904 - Management and control in leisure, display, therapy and other non-industrial systems is there to provide background and an overview of the risk of Legionella infection and how it can be controlled in leisure, display, therapy and other non-industrial water systems. It is a requirement of this course that candidates have successfully completed P901- Legionella- Management and Control of Building Hot and Cold Water Services [Syllabus GM.1]. Where both P901 and P904 courses are run on subsequent days or as a combined course then this pre-requirement is waived.

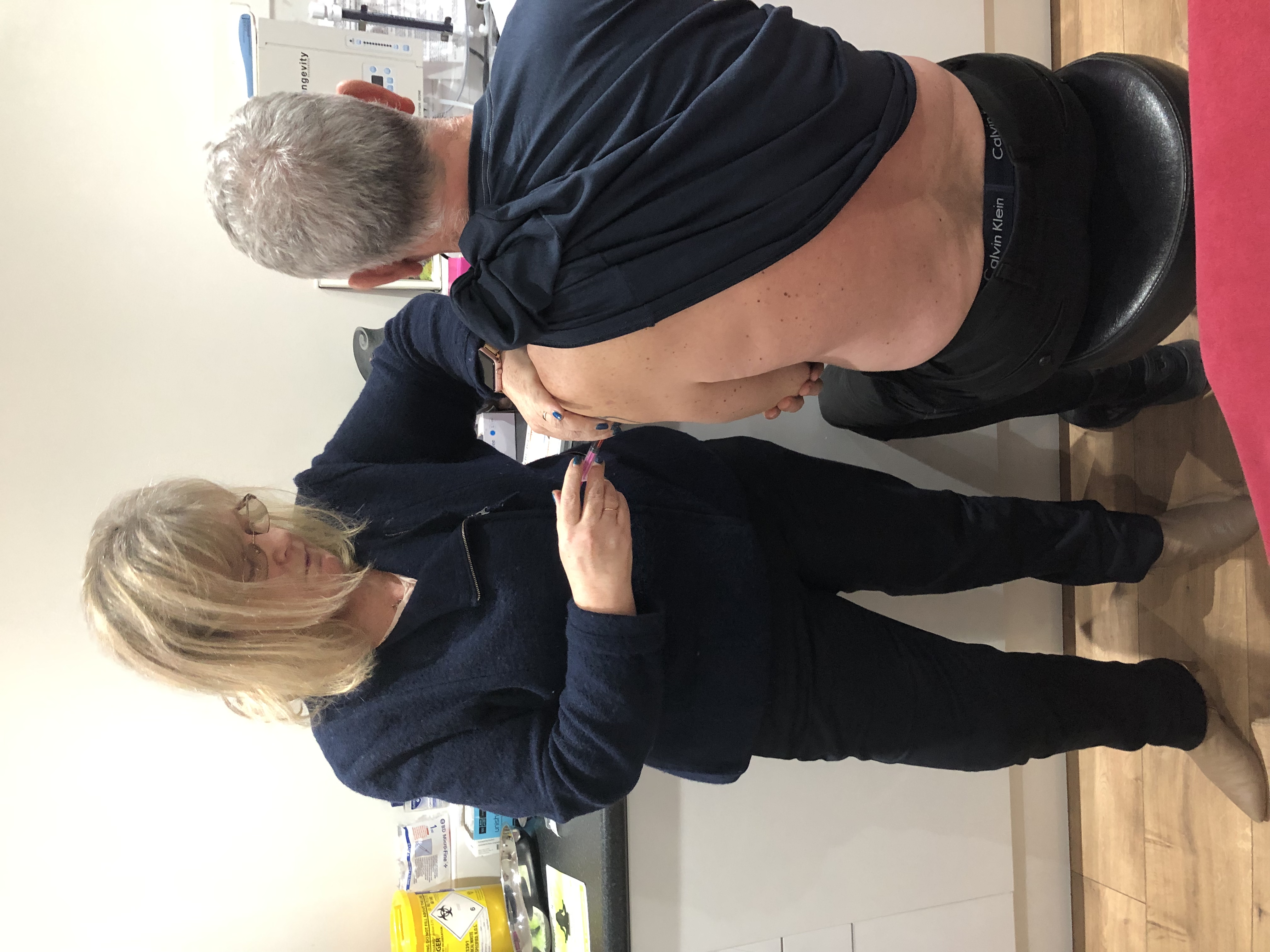
Course Introduction Covers B12 deficiency, pernicious anaemia, diagnosis, treatment and management. It also covers signs and symptoms Please note: this course is for health care professionals and nurses only. About this event Course Introduction This course concentrates on B12 deficiency, symptoms, treatments and management. The course covers B12 deficiency, pernicious anaemia, diagnosis, treatment and management. It also covers signs and symptoms of pernicious anaemia. This course is interactive and we include case studies and discuss issues regarding diagnostic testing. We review inclusion and exclusion criteria and identification of appropriate clients. Delegates will get the opportunity to review practice with hands on practical demonstrations of how to give injections correctly. We will cover administration techniques, where to give the injections and record keeping / documentation. We will discuss role and responsibilities and contraindications and precautions. The delegates will leave this course with an example of an individual protocol of Patient Specific Direction (PSD) and a competence based framework document to be used in practice. This course is very interactive. AIMS AND OBJECTIVES Understand the need for accountability and responsibility in relation to role development Demonstrate an understanding of safe practice Describe the signs and symptoms of pernicious anaemia Describe pernicious anaemia and its impact on patients Fully understand the principles, and practice B12 deficiency and B12 injections Understand the importance of safety issues related to giving injections Understand the law relating to role and function of the HCA and prescribing. Describe why patients require B12 injectionsBe able to correctly identify anatomical sites for injections Demonstrate correct administration techniquesDemonstrate how to correctly dispose of waste Demonstrate correct infection control procedures and use of PPE Describe when patients require referral and understand the importance of referral using correct clinical pathways Demonstrate an understanding of anaphylaxis and emergency procedures Understand the need for correct prescribing procedures Be able to document consultations following your organisations procedures COURSE CONTENTS Role and responsibilities Accountability guidelines and requirements Pernicious anaemia Blood- function B12 Deficiency Risk factors/groups Causes of B12 deficiency Diagnosis and reference ranges, testing Protocols and guidelines Factors affecting B12 diagnosis and treatment Factors affecting absorption B12 injections and common side effects Could it be B12 Deficiency Supplements Side effects and management including ADR’s Anaphylaxis Contraindications and Precautions Correct Administration and techniques including practical session Injection sites Legal Issues including consent Prescribing and Patient Specific Directions What to record Storage Disposal of injections/waste Infection control Needle stick injuries Competence and supervised practice Policies and procedures Facts and Figures Setting up and running a clinic Insurance/indemnity Research/evidence base and resources WHO SHOULD ATTEND? HCAs Nurses Doctors Pharmacists Anyone interested in Vitamin B12 deficiency and pernicious anaemia and those working with clients with B12 deficiency AB Health Group awards CPD points / certificate of attendance for each course. If you would prefer an accredited certificate by our accrediting body Aim Qualifications we can organise this. The charge for the certificate including postage is £30.
Quality Assurance for Good Laboratory Practice
By Research Quality Association
Course Information A must-have programme for Quality Assurance auditors stepping into or honing their role within a Good Laboratory Practice (GLP) environment, this course offers invaluable, expert guidance for crafting a robust and efficient GLP audit programme. What will I learn? A solid regulatory foundation underpinning quality assurance activities Clarity on the roles of Quality Assurance, management, and study director within the framework of Good Laboratory Practice principles Enhanced efficacy in inspections and audits Heightened compliance with Good Laboratory Practice standards for your facility Unique insights into governmental monitoring activities within the GLP sphere. This course is structured to encourage delegates to Discuss and develop ideas Solve specific problems Examine particular aspects of GLP. Tutors Tutors will be comprised of (click the photos for biographies): Cate Ovington Director, The Knowlogy Group Ltd Jane Elliston Senior Quality Assurance Auditor, Battelle UK Shona Ross Head of QA, Tower Mains Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Welcome and Introductions 09:15 Good Laboratory Practice Standards and Regulations An insight into the background and history of Good Laboratory Practice. 09:45 Principles of Quality Assurance What is the role and responsibilities of QA in GLP. Maintaining the independence of QA and what is an audit. 10:30 Break 10:45 Standard Operating Procedures GLP requirements and QA involvement. 11:30 Study Plans GLP requirements and QA involvement. 12:05 QA Programme Risk based programme, what are study, process and facility audits. 13:00 Lunch 14:00 Inspections Attitudes, techniques and attributes. 14:40 Workshop 1 - Facility and Process Inspections An exercise in inspection planning and preparation for inspections. 15:15 Break 15:30 Workshop 1 - Feedback 15:45 The Auditor and Audit Conduct Attitudes, attributes and techniques. 16:30 Panel Session An opportunity for delegates to put questions to the panel of speakers. 17:15 Close of Day Day 2 09:00 Workshop 2 - A Mock Audit 10:45 Break 11:00 Workshop 2 - Feedback 11:30 Auditing the Study Report Techniques and methods for the QA audit of the study report. 12:00 Record Keeping and Data The impact of GLP on data and records management. 12:40 Lunch 13:25 Data Integrity A look at the OECD GLP guidance document; the expectations of the regulators and the involvement of QA - Where QA adds value. 14:15 Workshop 3 - Amendments to Study Plan and Deviations from the Plan What are they? What is the difference between them? How are they controlled? 15:00 Workshop 3 - Feedback 15:15 Break 15:30 Regulatory Compliance GLP Monitoring Authority monitoring for compliance with Good Laboratory Practice. 16:15 Panel Session An opportunity for delegates to put questions to the panel of speakers. 16:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Learn

3-Day First Aid at Work Course
By Madeleys First Aid Plus
🩺 Be Prepared. Be Confident. Be a Lifesaver. 🩹 Is your workplace ready for emergencies? Our 3-day First Aid at Work qualification meets the legal requirements under the Health and Safety (First Aid) Regulations 1981 and (Northern Ireland) 1982. ✅ Gain essential knowledge ✅ Build practical first aid skills ✅ Be ready to respond in a wide range of workplace situations Perfect for: 🏢 Workplaces with identified first aid needs 🤝 Voluntary and community groups 🧑🤝🧑 Anyone responsible for first aid provision 📅 Don’t wait for an emergency to happen—get trained and qualified today! #FirstAid #WorkplaceSafety #HealthAndSafety #FirstAidTraining #BePrepared #LifesavingSkills

Management training for Stonehouse Care, to include Mental Capacity Act, Safeguarding and Health and Safety for Managers. The Mental Capacity Act & Deprivation of Liberty Safeguards for Managers and Senior Staff By the end of the course, you will be able to: Explain your legal responsibilities under the MCA and DoLS. Apply the five statutory principles of the MCA in decision-making. Determine when and how to conduct a capacity assessment. Understand the Best Interests process and how to apply it. Identify when a deprivation of liberty occurs and how to seek authorisation. Recognise Worcestershire-specific procedures and contacts for DoLS referrals. Implement MCA-compliant record-keeping practices. Safeguarding Adults & Children: Responsibilities for Health & Social Care Managers & Seniors By the end of the course, you will be able to: Understand their legal responsibilities under key safeguarding legislation. Recognise the roles and duties of managers and senior staff in safeguarding adults and children. Know Worcestershire's local safeguarding procedures and reporting pathways. Be able to apply best practices in safeguarding supervision, record-keeping, and decision-making. Identify when and how to escalate safeguarding concerns effectively. Health & Safety for Health & Social Care Managers and Seniors By the end of the course, you will be able to: Understand their legal responsibilities under Health & Safety legislation. Recognise how key regulations apply to their role in Health & Social Care. Learn how to manage risks and create a safe working environment. Be aware of local Worcestershire requirements and guidance. Identify best practices for compliance and enforcement.

Search By Location
- Record Keeping Courses in London
- Record Keeping Courses in Birmingham
- Record Keeping Courses in Glasgow
- Record Keeping Courses in Liverpool
- Record Keeping Courses in Bristol
- Record Keeping Courses in Manchester
- Record Keeping Courses in Sheffield
- Record Keeping Courses in Leeds
- Record Keeping Courses in Edinburgh
- Record Keeping Courses in Leicester
- Record Keeping Courses in Coventry
- Record Keeping Courses in Bradford
- Record Keeping Courses in Cardiff
- Record Keeping Courses in Belfast
- Record Keeping Courses in Nottingham

