- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
54 Consent courses in Cardiff delivered Live Online
Certified Information Privacy Manager (CIPM)
By Training Centre
Make a difference in your organization and in your career. The CIPM designation says that you're a leader in privacy program administration and that you've got the goods to establish, maintain and manage a privacy program across all stages of its lifecycle. The CIPM is the world's first and only certification in privacy program management. When you earn a CIPM, it shows that you don't just know privacy regulations-you know how to make it work for your organization. In other words, you're the go-to person for day-to-day operations when it comes to privacy. Developed in collaboration with the law firms, Bird and Bird, Field Fisher, Wilson/Sonsini and Covington and Burling, the CIPM encompasses pan-European and national data protection laws, key privacy terminology and practical concepts concerning the determination of control measures designed to protect personal data and trans-border data flows. About This Course Delivered in a modular format, the course covers; Module 1: Introduction to privacy program management Identifies privacy program management responsibilities, and describes the role of accountability in privacy program management. Module 2: Privacy governance Examines considerations for developing and implementing a privacy program, including the position of the privacy function within the organization, role of the DPO, program scope and charter, privacy strategy, support and ongoing involvement of key functions and privacy frameworks. Module 3: Applicable laws and regulations Discusses the regulatory environment, common elements across jurisdictions and strategies for aligning compliance with organizational strategy. Module 4: Data assessments Relates practical processes for creating and using data inventories/maps, gap analyses, privacy assessments, privacy impact assessments/data protection impact assessments and vendor assessments. Module 5: Policies Describes common types of privacy-related policies, outlines components and offers strategies for implementation. Module 6: Data subject rights Discusses operational considerations for communicating and ensuring data subject rights, including privacy notice, choice and consent, access and rectification, data portability, and erasure and the right to be forgotten. Module 7: Training and awareness Outlines strategies for developing and implementing privacy training and awareness programs. Module 8: Protecting personal information Examines a holistic approach to protecting personal information through privacy by design. Module 9: Data breach incident plans Provides guidance on planning for and responding to a data security incident or breach. Module 10: Measuring, monitoring and auditing program performance Relates common practices for monitoring, measuring, analyzing and auditing privacy program performance The CIPM body of knowledge outlines all the concepts and topics that you need to know to become certified. The exam blueprint gives you an idea of how many questions from each topic area you can expect on the exam. Prerequisites There are no prerequisites for this course but attendees would benefit from a review of the materials on the IAPP site What's Included? Breakfast, Lunch and refreshments (Classroom based courses only) The Official Study Guide (Privacy Programme Management - Third Edition)* Official Exam Q&A's* Official Practice Exam Participant Guide* 1 years membership of the IAPP The Exam Fees * In electronic format for Live Online and hard copy for Classroom delegates Who Should Attend? The CIPM is ideal for IT and information security leaders responsible for developing Data Protection frameworks and applying best practice, including those in the following positions: Data Analysts Cyber Security Analysts and Managers Aspiring Data Protection Officers IT Managers Accreditation Our Guarantee We are an approved IAPP Training Partner. You can learn wherever and whenever you want with our robust classroom and interactive online training courses. Our courses are taught by qualified practitioners with a minimum of 25 years commercial experience. We strive to give our delegates the hands-on experience. Our courses are all-inclusive with no hidden extras. The one-off cost covers the training, all course materials, and exam voucher. Our aim: To achieve a 100% first time pass rate on all our instructor-led courses. Our Promise: Pass first time or 'train' again for FREE. *FREE training offered for retakes - come back within a year and only pay for the exam.

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

AZ-204T00 Developing Solutions for Microsoft Azure
By Nexus Human
Duration 5 Days 30 CPD hours This course is intended for Students in this course are interested in Azure development or in passing the Microsoft Azure Developer Associate certification exam. This course teaches developers how to create end-to-end solutions in Microsoft Azure. Students will learn how to implement Azure compute solutions, create Azure Functions, implement and manage web apps, develop solutions utilizing Azure storage, implement authentication and authorization, and secure their solutions by using KeyVault and Managed Identities. Students will also learn how to connect to and consume Azure services and third-party services, and include event- and message-based models in their solutions. The course also covers monitoring, troubleshooting, and optimizing Azure solutions. Prerequisites To be successful in this course, learners should have the following: Hands-on experience with Azure IaaS and PaaS solutions, and the Azure Portal. Experience writing in an Azure supported language at the intermediate level. (C#, JavaScript, Python, or Java) Ability to write code to connect and perform operations on, a SQL or NoSQL database product. (SQL Server, Oracle, MongoDB, Cassandra or similar) Experience writing code to handle authentication, authorization, and other security principles at the intermediate level. A general understanding of HTML, the HTTP protocol and REST API interfaces. 1 - Explore Azure App Service Examine Azure App Service Examine Azure App Service plans Deploy to App Service Explore authentication and authorization in App Service Discover App Service networking features 2 - Configure web app settings Configure application settings Configure general settings Configure path mappings Enable diagnostic logging Configure security certificates 3 - Scale apps in Azure App Service Examine autoscale factors Identify autoscale factors Enable autoscale in App Service Explore autoscale best practices 4 - Explore Azure App Service deployment slots Explore staging environments Examine slot swapping Swap deployment slots Route traffic in App Service 5 - Explore Azure Functions Discover Azure Functions Compare Azure Functions hosting options Scale Azure Functions 6 - Develop Azure Functions Explore Azure Functions development Create triggers and bindings Connect functions to Azure services 7 - Explore Azure Blob storage Explore Azure Blob storage Discover Azure Blob storage resource types Explore Azure Storage security features Discover static website hosting in Azure Storage 8 - Manage the Azure Blob storage lifecycle Explore the Azure Blob storage lifecycle Discover Blob storage lifecycle policies Implement Blob storage lifecycle policies Rehydrate blob data from the archive tier 9 - Work with Azure Blob storage Explore Azure Blob storage client library Create a client object Manage container properties and metadata by using .NET Set and retrieve properties and metadata for blob resources by using REST 10 - Explore Azure Cosmos DB Identify key benefits of Azure Cosmos DB Explore the resource hierarchy Explore consistency levels Choose the right consistency level Explore supported APIs Discover request units 11 - Work with Azure Cosmos DB Explore Microsoft .NET SDK v3 for Azure Cosmos DB Create stored procedures Create triggers and user-defined functions Explore change feed in Azure Cosmos DB 12 - Manage container images in Azure Container Registry Discover the Azure Container Registry Explore storage capabilities Build and manage containers with tasks Explore elements of a Dockerfile 13 - Run container images in Azure Container Instances Explore Azure Container Instances Run containerized tasks with restart policies Set environment variables in container instances Mount an Azure file share in Azure Container Instances 14 - Implement Azure Container Apps Explore Azure Container Apps Explore containers in Azure Container Apps Implement authentication and authorization in Azure Container Apps Manage revisions and secrets in Azure Container Apps Explore Dapr integration with Azure Container Apps 15 - Explore the Microsoft identity platform Explore the Microsoft identity platform Explore service principals Discover permissions and consent Discover conditional access 16 - Implement authentication by using the Microsoft Authentication Library Explore the Microsoft Authentication Library Initialize client applications 17 - Implement shared access signatures Discover shared access signatures Choose when to use shared access signatures Explore stored access policies 18 - Explore Microsoft Graph Discover Microsoft Graph Query Microsoft Graph by using REST Query Microsoft Graph by using SDKs Apply best practices to Microsoft Graph 19 - Implement Azure Key Vault Explore Azure Key Vault Discover Azure Key Vault best practices Authenticate to Azure Key Vault 20 - Implement managed identities Explore managed identities Discover the managed identities authentication flow Configure managed identities Acquire an access token 21 - Implement Azure App Configuration Explore the Azure App Configuration service Create paired keys and values Manage application features Secure app configuration data 22 - Explore API Management Discover the API Management service Explore API gateways Explore API Management policies Create advanced policies Secure APIs by using subscriptions Secure APIs by using certificates 23 - Explore Azure Event Grid Explore Azure Event Grid Discover event schemas Explore event delivery durability Control access to events Receive events by using webhooks Filter events 24 - Explore Azure Event Hubs Discover Azure Event Hubs Explore Event Hubs Capture Scale your processing application Control access to events Perform common operations with the Event Hubs client library 25 - Discover Azure message queues Choose a message queue solution Explore Azure Service Bus Discover Service Bus queues, topics, and subscriptions Explore Service Bus message payloads and serialization Explore Azure Queue Storage Create and manage Azure Queue Storage and messages by using .NET 26 - Monitor app performance Explore Application Insights Discover log-based metrics Instrument an app for monitoring Select an availability test Troubleshoot app performance by using Application Map 27 - Develop for Azure Cache for Redis Explore Azure Cache for Redis Configure Azure Cache for Redis Interact with Azure Cache for Redis by using .NET 28 - Develop for storage on CDNs Explore Azure Content Delivery Networks Control cache behavior on Azure Content Delivery Networks Interact with Azure Content Delivery Networks by using .NET

SOSAFE! SOCIAL & SEXUAL SAFETY
By Pyramid Educational Consultants Uk, Ltd.
Do you ever worry about how safe your students are in their relationships? Do you think that they might be vulnerable to abuse? You may work with students who indiscriminately sit on people’s laps or hug a stranger; or alternatively an adult with learning difficulties who is capable of having close intimate connections, but you worry about the speed of which they enter these relationships. These are common concerns and situations for any parent or care giver. It is well acknowledged that this need; to educate children and young people about relationships and social safety, is not being met at the moment. The SoSAFE! Programme is a visual teaching tool which enables learners to develop their abilities in managing; and communicating about their relationships. The program covers all degrees of interaction; from a student’s expectations of strangers, to the intricacies of an intimate relationship. SoSAFE! moves away from making feelings based decisions so we can enable and empower our learners to make their own judgments about what is okay to do with specific people. SoSAFE! provides a format which is portable and easy to understand, meaning that any student can discuss their relationships at any time. By giving students a set of ‘rules’ for different relationships they are better placed to realise when a relationship may not be acceptable and then have the tools to report potential abuse. If you want your students to have more success in safer relationships; but there is a gap in your knowledge that prevents you from doing this, then SoSAFE! is the framework for you. Teach them to be SoSAFE! Read a blog about one school's experience of implementing the programme. WHAT YOU WILL LEARN Discuss the history of and reasons for teaching social safety Identify characteristics of students and how best to support them Explore how to practically implement the SoSAFE! tool kit Learn how to teach explicit consent to any user Discover how to apply SoSAFE! to real life scenarios You can find further information on the SoSAFE Programme at sosafeprogram.com WORKSHOP DETAILS Agenda: 9:00 AM - 4:00 PM Registration Time: 8:30 AM - 9:00 AM Tuition Includes: A SoSAFE! Manual, all programme materials and SoSAFE! Tool Kit on a USB stick, handout with space for note taking and Certificate of Attendance.

European Data Protection & Privacy Programme Management - DPO Ready
By Training Centre
European Data Protection Principles of Data Protection in Europe covers the essential pan-European and national data protection laws, as well as industry-standard best practices for corporate compliance with these laws. Those taking this course will gain an understanding of the European model for privacy enforcement, key privacy terminology and practical concepts concerning the protection of personal data and trans-border data flows. The training is based on the body of knowledge for the IAPP's ANSI-accredited Certified Information Privacy Professional/Europe (CIPP/E) certification program. Privacy Programme Management Principles of Privacy Management is the how-to training on implementing a privacy program framework, managing the privacy program operational lifecycle and structuring a knowledgeable, high-performing privacy team. Those taking this course will learn the skills to manage privacy in an organisation through process and technology-regardless of jurisdiction or industry. The Principles of Privacy Program Management training is based on the body of knowledge for the IAPP's ANSI-accredited Certified Information Privacy Manager (CIPM) certification programme. Make a difference in your organization and in your career. The CIPM designation says that you're a leader in privacy program administration and that you've got the goods to establish, maintain and manage a privacy program across all stages of its lifecycle. About This Course Delivered in a modular format, this four day course covers Days 1 & 2 Module 1: Data Protection Laws Introduces key European data protection laws and regulatory bodies, describing the evolution toward a Harmonised European Legislative Framework. Module 2: Personal Data Defines and differentiates between types of data-including personal, anonymous, pseudo-anonymous and special categories. Module 3: Controllers and Processors Describes the roles and relationships of controllers and processors. Module 4: Processing Personal Data Defines data processing and GDPR processing principles, Explains the application of the GDPR and outlines the legitimate bases for processing personal data. Module 5: Information provision Explains controller obligations for providing information about data processing activities to data subjects and Supervisory Authorities. Module 6: Data Subjects 'Rights Describes data subjects' rights, applications of rights and obligations controller and processor. Module 7: Security or Processing Discusses considerations and duties of controllers and processors for Ensuring security of personal data and providing notification of data breaches. Module 8: Accountability Investigates accountability requirements, data protection management systems, data protection impact assessments, privacy policies and the role of the data protection officer. Module 9: International Data Transfers Outlines options and obligations for transferring data outside the European Economic Area, Decisions adequacy and appropriateness safeguards and derogations. Module 10: Supervision and Enforcement Describes the role, powers and procedures or Supervisory Authorities; the composition and tasks of the European Data Protection Board; the role of the European Data Protection Supervisor; and remedies, liabilities and penalties for non-compliance. Module 11: Compliance Discusses the applications of European data protection law, legal bases and compliance requirements for processing personal data in practice, employers-including processing employee data, surveillance, direct marketing, Internet technology and communications and outsourcing. Days 3 & 4 Module 1: Introduction to privacy program management Identifies privacy program management responsibilities, and describes the role of accountability in privacy program management. Module 2: Privacy governance Examines considerations for developing and implementing a privacy program, including the position of the privacy function within the organization, role of the DPO, program scope and charter, privacy strategy, support and ongoing involvement of key functions and privacy frameworks. Module 3: Applicable laws and regulations Discusses the regulatory environment, common elements across jurisdictions and strategies for aligning compliance with organizational strategy. Module 4: Data assessments Relates practical processes for creating and using data inventories/maps, gap analyses, privacy assessments, privacy impact assessments/data protection impact assessments and vendor assessments. Module 5: Policies Describes common types of privacy-related policies, outlines components and offers strategies for implementation. Module 6: Data subject rights Discusses operational considerations for communicating and ensuring data subject rights, including privacy notice, choice and consent, access and rectification, data portability, and erasure and the right to be forgotten. Module 7: Training and awareness Outlines strategies for developing and implementing privacy training and awareness programs. Module 8: Protecting personal information Examines a holistic approach to protecting personal information through privacy by design. Module 9: Data breach incident plans Provides guidance on planning for and responding to a data security incident or breach. Module 10: Measuring, monitoring and auditing program performance Relates common practices for monitoring, measuring, analyzing and auditing privacy program performance Prerequisites There are no prerequisites for this course but attendees would benefit from a review of the materials on the IAPP SITE What's Included? 1 years membership of the IAPP Breakfast, Lunch, mid-morning and afternoon snacks, teas, coffees Official Study Guides* Official Participant Guides* Official Exam Q&A's* Both exam fees * In electronic format for Live Online and hard copy for Classroom delegates Who Should Attend? This course is suitable for aspiring Data Protection Officers, as well as Information Security Managers, Lawyers, Data Managers, Analysts and Risk Teams. Provided by Our Guarantee We are an approved IAPP Training Partner. You can learn wherever and whenever you want with our robust classroom and interactive online training courses. Our courses are taught by qualified practitioners with a minimum of 25 years commercial experience. We strive to give our delegates the hands-on experience. Our courses are all-inclusive with no hidden extras. The one-off cost covers the training, all course materials, and exam voucher. Our aim: To achieve a 100% first time pass rate on all our instructor-led courses. Our Promise: Pass first time or 'train' again for FREE. *FREE training offered for retakes - come back within a year and only pay for the exam.

BRAZILIAN BUTTOCK LIFT (BBL TRAINING COURSE)
By Harley Elite Academy (HeLa)
MASTERCLASS ONE-2-ONE 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! This course is intended for delegates who are now doing dermal fillers or whom are advanced injectors. The Brazilian Butt Lift training includes improving shape, volumizing and sculpting the area with dermal filler. We use Hyacorp MFL 2 or Genefill Products During the training day you will learn the following: Anatomy and Physiology Consultation for patients Consent forms Treatment information After care Dezinfection & Safety Indications and Contra-indications Risks, side effects Dealing with complications Marketing tips Certificate upon completion CPD All under the supervision of our fully trained aesthetics practitioners, with Medical background .You will practice on real models and optional extra Mentoring date can be provided if required! BBL is the newest of the body augmentation treatments in the cosmetic/aesthetic industry Masterclass. BBL TRAINING COURSE IN LONDON Practice in 1-day Course ONE-TO-ONE Training Non-Surgical Liquid Brazilian Butt Lift with Hyaluronic Acid ( difference Fillers )Hayacorp MFL 2 ,Genefill contour using cannulas. Brazilian Butt lift with Fillers also called Liquid BBL has become one of the most popular non-surgical cosmetic procedures. Client’s are more than ever looking for non-surgical alternatives to the most popular cosmetic procedures. Take advantage of this ever-growing market with our one-day intensive course, extend you level of injectable procedure. Our Buttock Augmentation training course you will learn how to safely administrate the HA and effectively carry out these procedures to help your clients achieve a firmer, rounder buttock whilst saving your clients thousands on undergoing invasive surgery. The Brazilian Buttock Lift, training course consists in learning Master class injection techniques and application for the most frequently requested areas of the body under professional supervise with some of the best experts of Academy on central London. You can extend the training with Legs, Arms and Calves. With Harley Elite Academy you can learn to practice in cosmetic medicine what the most celebrities want! We will cover pertinent information including mechanism of action technique of safety, dealing with complications, dilution guidelines, and more., providing injectable protocol for the products. Contraindications Complications Management Post treatment advice You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (theory), IN-CLINIC (Practice) BBL AREA BBL | Filler, BBL | Sculpture, BBL | PDO Cogs Threads COURSE LEVEL EXPERT | Masterclass Course
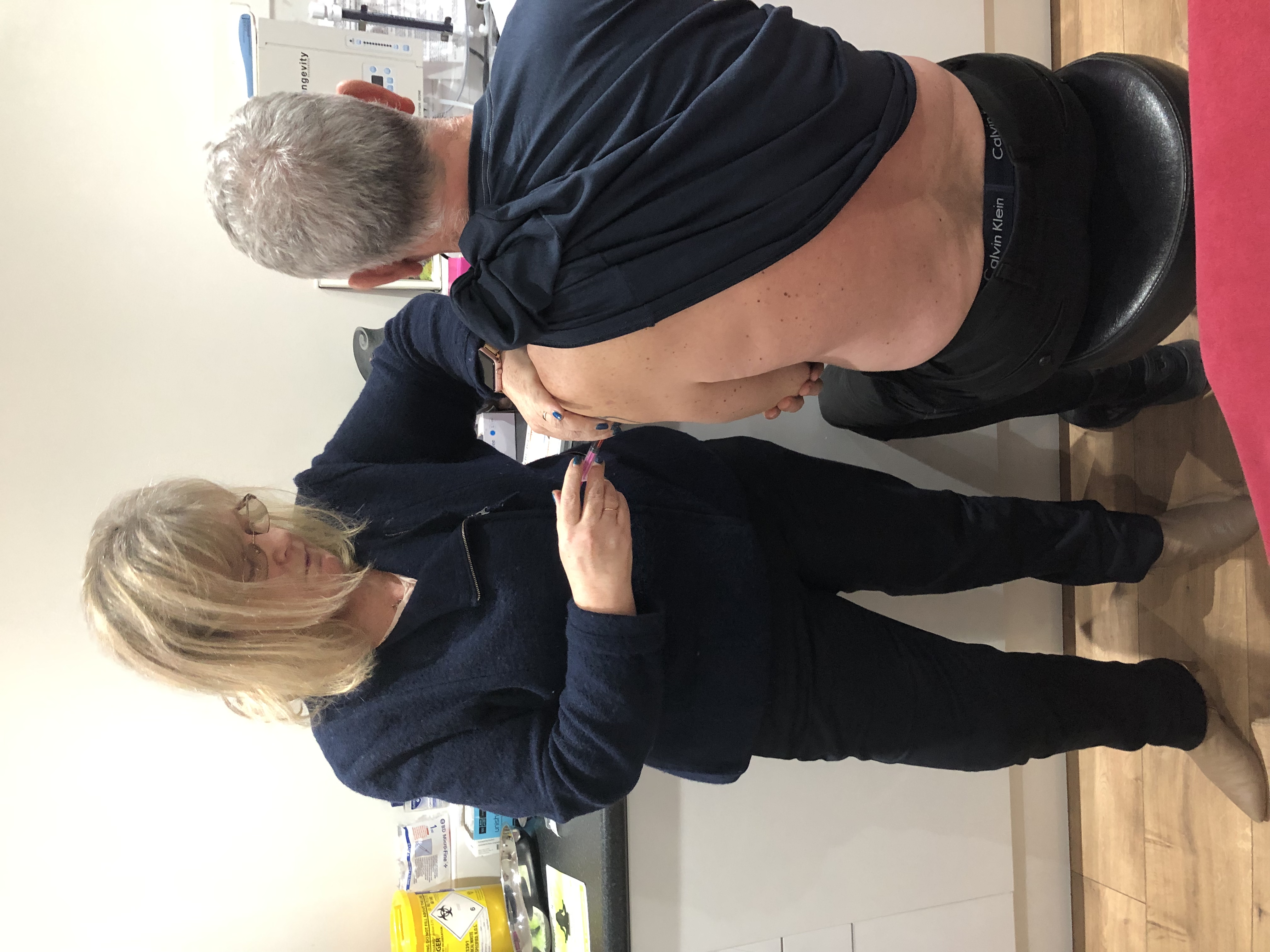
Immunisation Update - National Cohort Immunisations (Adult/Child)
By BBO Training
Half Day Update in National Cohort Immunisations (Adult/Child)This half-day update course offers a focused and comprehensive review of national cohort immunisations for both adults and children. Designed as an annual update for professionals who have already completed a two-day foundation course, this session aligns with the recommendations by Public Health England. The course content fulfills the mandatory requirements outlined in the Public Health England (2018) National Minimum Standards and Core Curriculum for Registered Healthcare Practitioners. Agenda: 09:00: Registration 09:10: Introduction and Course Objectives 09:45: Current Issues in Vaccination 10:00: Changes/Updates to National Immunisation Schedules for Adults and Children 10:45: Coffee Break 11:00: Quality and Outcomes Framework (QOF) Updates 11:20: Cold Chain Management 11:40: Anaphylaxis Overview 11:55: Review of Current Practice - Identifying Opportunities for Improvement 12:10: Question and Answer Session for Shared Learning and Addressing Common Problems in Primary Care Learning Outcomes:By the conclusion of this half-day update course, participants will be able to:1. Comprehend Legal Aspects of Vaccination Delivery: - Understand the legal and regulatory framework surrounding the administration of vaccinations, ensuring compliance with relevant guidelines and regulations.2. Review Best Practices for Cold Chain and Vaccine Storage: - Assess and reinforce proper procedures for maintaining the cold chain, ensuring the safety and efficacy of vaccines through meticulous storage practices.3. Enhance Risk Assessment Skills for Safe Vaccination Programs: - Review and apply robust risk assessment methodologies to ensure the secure and effective delivery of vaccination programs.4. Provide Current Vaccine and Disease Information: - Demonstrate an up-to-date knowledge of vaccines, their indications, contraindications, and the diseases they protect against, enabling informed and accurate patient education.5. Encourage and Explore Accurate Documentation: - Promote the importance of accurate and comprehensive documentation throughout the vaccination process, including consent forms, vaccine records, and adverse event reporting.6. Foster Discussion of Practice Challenges and Solutions: - Create an environment for open dialogue and collaborative learning, facilitating the exchange of experiences, challenges, and effective solutions within the realm of cohort immunisations.This concise update session is designed to reinforce foundational knowledge, ensure compliance with current standards, and empower healthcare professionals to continue delivering safe, effective, and informed vaccination services.
FAT DISOLVING | Aqualyx or Lemon Bottle
By Harley Elite Academy (HeLa)
ADVANCED 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! Lipolysis (Fat Disolving) achieves good results in the following body zones: – Chin (double chin) – Hips (love handles) – Stomach (abdominal area) – Thighs (saddlebags) – Upper arms (arm toning) – Pseudo gynecomastia (male breasts) – Back (bra fat or muffin top) The acids present in Aqualyx cause fat destruction in the body. Aqualyx main active ingredient is deoxycholic acid. Deoxycholic acid is a bile acid, synthesized in the human liver. The fatty acids are then released into the body, to be broken down by our usual metabolic processes in the liver. Naturally occurring bile acid is used by the body to emulsify fat. Aqualyx comes in a water based injection that dissolves fat cells that it comes into contact with. A single vial will be sufficient. Small are for larger areas, such as the abdomen or the inner thigh, anywhere between 5 to 10 vials One treatment of AQUALYX® usually includes only 1 or 2 injection sites, as well as a local anaesthetic solution of lidocaine which is used to irradicate any pain and make the procedure as comfortable as possible. Course Content Disinfection, Health & Safety. Consultation and timings including data protection, medical history and client consent Skin Types Skin analysis Pre and post treatment procedures Injection protocol and techniques Safe handling needles, before, during and after treatment Product knowledge Setting up Treatment procedure Results clients can expects and managing expectations Contractions and aftercare advice Treatment planning and pricing Post care instruction Fat dissolving products (Lemon Bottle), very safe. Lemon Bottle is a high-concentration fat dissolve solution that combines Riboflavin (vitamin B2) and other premium ingredients that create fat decomposition by accelerating metabolism of fat cells. Become a certified Lemon Bottle fat-dissolving treatments expert with our comprehensive and accredited courses designed specifically for UK practitioners. We have the perfect course to suit your needs, focusing on the Lemon Bottle system. Minimal swelling, minimal pain. Begins working immediately. You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (Theory), IN CLINIC (Practice) COURSE LEVEL INTERMEDIATE | Advanced Course

Course Introduction Covers B12 deficiency, pernicious anaemia, diagnosis, treatment and management. It also covers signs and symptoms. This course does cover how to set up your own service. Please note: this course is for health care professionals or those working in Primary Care. It is not open to Beauticians unless you are a registered healthcare professional. If in doubt- please email annie at training@anniebarr.com or phone +44 7500048222 About this event Course Introduction This course concentrates on B12 deficiency, symptoms, treatments and management. The course covers B12 deficiency, pernicious anaemia, diagnosis, treatment and management. It also covers signs and symptoms of pernicious anaemia. This course is interactive and we include case studies and discuss issues regarding diagnostic testing. We review inclusion and exclusion criteria and identification of appropriate clients. Delegates will get the opportunity to review practice with hands on practical demonstrations of how to give injections correctly. We will cover administration techniques, where to give the injections and record keeping / documentation. We will discuss role and responsibilities and contraindications and precautions. The delegates will leave this course with an example of an individual protocol of Patient Specific Direction (PSD) and a competence based framework document to be used in practice. This course is very interactive. AIMS AND OBJECTIVES Understand the need for accountability and responsibility in relation to role development Demonstrate an understanding of safe practice Describe the signs and symptoms of pernicious anaemia Describe pernicious anaemia and its impact on patients Fully understand the principles, and practice B12 deficiency and B12 injections Understand the importance of safety issues related to giving injections Understand the law relating to role and function of the HCA and prescribing. Describe why patients require B12 injectionsBe able to correctly identify anatomical sites for injectionsDemonstrate correct administration techniquesDemonstrate how to correctly dispose of wasteDemonstrate correct infection control procedures and use of PPEDescribe when patients require referral and understand the importance of referral using correct clinical pathwaysDemonstrate an understanding of anaphylaxis and emergency proceduresUnderstand the need for correct prescribing proceduresBe able to document consultations following your organisations procedures COURSE CONTENTS Role and responsibilities Accountability guidelines and requirements Pernicious anaemia Blood- function B12 Deficiency Risk factors/groups Causes of B12 deficiency Diagnosis and reference ranges, testing Protocols and guidelines Factors affecting B12 diagnosis and treatment Factors affecting absorption B12 injections and common side effects Could it be B12 Deficiency Supplements Side effects and management including ADR’s Contraindications and Precautions Correct Administration and techniques including practical session Injection sites Legal Issues including consent Prescribing and Patient Specific Directions What to record Storage Disposal of injections/waste Infection control Needle stick injuries Competence and supervised practice Policies and procedures Facts and Figures Setting up and running a clinic Insurance/indemnity Research/evidence base and resources Please note Anaphylaxis is not covered on this course, however we do run a separate Anaphylaxis 1hr training course, which takes place at the end of this B12 webinar. If you wish to do the 1 hr Anaphylaxis also, you should book onto BOTH courses. WHO SHOULD ATTEND? HCAs Nurses Doctors Pharmacists Anyone interested in Vitamin B12 deficiency and pernicious anaemia and those working with clients with B12 deficiency AB Health Group awards CPD points / certificate of attendance for each course. If you would prefer an accredited certificate by our accrediting body Aim Qualifications we can organise this. The charge for the certificate including postage is £30.