- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
103 Clinical Research courses in Cardiff delivered Online
Good Clinical Practices: A Practical Guide to GCP Compliance
By Xpert Learning
About Course Understand the Ethical and Regulatory Framework for Conducting Clinical Trials Course Description This comprehensive Good Clinical Practices (GCP) course provides a thorough understanding of the ethical and regulatory principles governing clinical trials. It delves into the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines, ensuring you grasp the essential standards for conducting clinical research. Course Objectives By the end of this course, you will be able to: Articulate the definition, purpose, and historical context of GCP. Explain the importance of GCP in protecting human subjects and ensuring data integrity. Identify key international organizations involved in establishing GCP standards. Apply ethical principles and informed consent procedures in clinical research. Understand the ICH E6(R2) guidelines on Integrated Addendum to Good Clinical Practice. Design and conduct clinical trials according to ICH E8 and E9 guidelines. Manage and report clinical trial data in compliance with ICH E6(R2) guidelines. Implement safety monitoring and adverse event reporting procedures as per ICH E6(R2) and E6(R3) guidelines. Navigate regulatory compliance and inspections related to clinical trials. Target Audience This course is designed for individuals who: Are interested in pursuing a career in clinical research or clinical trial management. Work in the pharmaceutical, biotechnology, or medical device industry. Seek to gain a comprehensive understanding of GCP principles and practices. Prerequisites No prior experience in clinical research is required. However, a basic understanding of research methodology and medical terminology is recommended. Please Note: This course is strictly theoretical and does not qualify participants for clinical practice in the industry. Additional training and certifications may be required for hands-on experience. What Will You Learn? Articulate the definition, purpose, and historical context of GCP. Explain the importance of GCP in protecting human subjects and ensuring data integrity. Identify key international organizations involved in establishing GCP standards. Apply ethical principles and informed consent procedures in clinical research. Understand the ICH E6(R2) guidelines on Integrated Addendum to Good Clinical Practice. Design and conduct clinical trials according to ICH E8 and E9 guidelines. Manage and report clinical trial data in compliance with ICH E6(R2) guidelines. Implement safety monitoring and adverse event reporting procedures as per ICH E6(R2) and E6(R3) guidelines. Navigate regulatory compliance and inspections related to clinical trials. Course Content Introduction to Good Clinical Practice (GCP) and ICH Guidelines Introduction to Good Clinical Practice (GCP) and ICH Guidelines Ethical Principles, Informed Consent, and ICH E6(R2) Ethical Principles, Informed Consent, and ICH E6(R2) Designing and Conducting Clinical Trials with ICH E8 and E9 Designing and Conducting Clinical Trials with ICH E8 and E9 Data Management and Reporting with ICH E6(R2) Data Management and Reporting with ICH E6(R2) Safety and Monitoring in Clinical Trials with ICH E6(R2) and E6(R3) Safety and Monitoring in Clinical Trials with ICH E6(R2) and E6(R3) Regulatory Compliance, Inspections, and ICH E6(R3) Regulatory Compliance, Inspections, and ICH E6(R3) A course by Xpert Learning RequirementsA basic understanding of research methodology and medical terminology is recommended. Audience Individuals who are interested in pursuing a career in clinical research or clinical trial management. Individuals who work in the pharmaceutical, biotechnology, or medical device industry. Individuals who seek to gain a comprehensive understanding of GCP principles and practices. Audience Individuals who are interested in pursuing a career in clinical research or clinical trial management. Individuals who work in the pharmaceutical, biotechnology, or medical device industry. Individuals who seek to gain a comprehensive understanding of GCP principles and practices.

The healthcare sector is not only about treatment and care—it’s a vast, structured system built on financial strategy, data governance, and operational efficiency. The Business of Health Care Course is designed for those interested in understanding the frameworks behind healthcare organisations, from how services are managed to how funding and research are coordinated. This course blends key aspects of healthcare business strategy, patient service, and data security, offering a modern outlook on how health and care providers function behind the scenes. Covering everything from GDPR and information governance to budgeting, insurance, and clinical research administration, this course presents a rounded view of healthcare as a business. Whether you're looking to move into healthcare management or simply want to better understand the systems that support care delivery, this course provides essential knowledge that connects healthcare operations with financial and organisational decision-making. It's a smart choice for those looking to gain insight into the business side of a complex and constantly evolving industry. This The Business of Health Care bundle contains the following courses : Course 01: Healthcare GDPR Course 02: Business and Management Course 03: Financial Management Course 04: Budgeting Course 05: Social Media for Health & Care Course 06: Health Science Course 07: Clinical Research Administration Fundamentals Course 08: Information Governance Course 09: Patient Customer Service Course 10: Health Economics and Health Technology Course 11: Insurance Key Features: CPD Certified Instant e-certificate and hard copy dispatch by next working day Fully online, interactive course with audio voiceover Developed by qualified professionals in the field Self-paced learning and laptop, tablet, smartphone-friendly 24/7 Learning Assistance Discounts on bulk purchases Here are brief descriptions for each course in the "The Business of Health Care" bundle: Healthcare GDPR : Gain a comprehensive understanding of the General Data Protection Regulations (GDPR) within the healthcare context. Learn how to navigate the legal framework, ensure compliance, and protect sensitive medical information. Business Management : Explore the fundamentals of strategic management in business, from corporate governance to building a brand. Discover how to formulate and implement effective strategies, evaluate performance, and foster strong customer relationships. Financial Management : Master the essentials of financial management, including budgeting, interpreting financial statements, and analyzing cash flow. Understand the international aspects of financial management for effective decision-making. Budgeting : Learn the intricacies of budgeting, from understanding its importance to the detailed process of creating and managing budgets effectively. Social Media for Health & Care : Discover the role of social media in healthcare communication, big data utilization, and participatory health. Explore strategies for leveraging social media platforms to promote health behavior change and engage with patients. Health Science : Delve into the core principles of health science, covering anatomy, physiology, epidemiology, and healthcare delivery systems. Enhance critical thinking skills and gain insights into public health challenges. Clinical Research Administration Fundamentals : Understand the fundamentals of clinical research administration, from trial design to data management and regulatory compliance. Explore the future trends shaping clinical research practices. Information Governance : Learn the essentials of information governance, including data protection, records management, and cybersecurity. Develop strategies to ensure privacy, confidentiality, and compliance with regulatory requirements. Patient Customer Service : Enhance your patient customer service skills with modules covering effective training, operative skills, and handling difficult situations. Learn to provide exceptional service and support within medical settings. Health Economics and Health Technology : Explore the intersection of health economics and technology assessment, evaluating economic implications and policy decisions. Understand the role of health insurance in shaping healthcare systems. Insurance : Gain insights into the insurance industry, from principles and types of insurance to underwriting processes and fraud prevention. Understand the dynamics of both personal and commercial insurance sectors. Learning Outcomes: Understand Healthcare GDPR compliance and its implications for businesses. Apply principles of Business Management to healthcare organisations effectively. Analyse Financial Management strategies within the healthcare sector proficiently. Develop proficient Budgeting skills tailored for healthcare institutions. Utilise Social Media for Health & Care promotion and engagement effectively. Comprehend Health Science principles and their application in healthcare management. Administer Clinical Research according to established regulations and guidelines. Accreditation All of our courses, including the The Business of Health Care Bundle are fully accredited, providing you with up-to-date skills and knowledge and helping you to become more competent and effective in Financial Fraud Detection. Certification Once you've successfully completed your The Business of Health Care Bundle, you will immediately be sent your digital certificates. Also, you can have your printed certificate delivered by post (shipping cost £3.99). Our The Business of Health Care certification have no expiry dates, although we recommend renewing them every 12 months. CPD 110 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Healthcare professionals seeking business management skills enhancement. Individuals aspiring for administrative roles within healthcare organisations. Graduates with a healthcare background interested in business aspects. Entrepreneurs aiming to establish healthcare-related ventures or startups. Managers in healthcare settings requiring broader business acumen. Requirements There are no formal entry requirements for the course, with enrollment open to anyone! Career path Healthcare Administrator Health Service Manager Healthcare Finance Analyst Clinical Research Coordinator Health Information Manager Certificates Digital certificate Digital certificate - Included Once you've successfully completed your course, you will immediately be sent a FREE digital certificate. Hard copy certificate Hard copy certificate - Included Also, you can have your FREE printed certificate delivered by post (shipping cost £3.99 in the UK). For all international addresses outside of the United Kingdom, the delivery fee for a hardcopy certificate will be only £10. Our certifications have no expiry dates, although we do recommend that you renew them every 12 months.

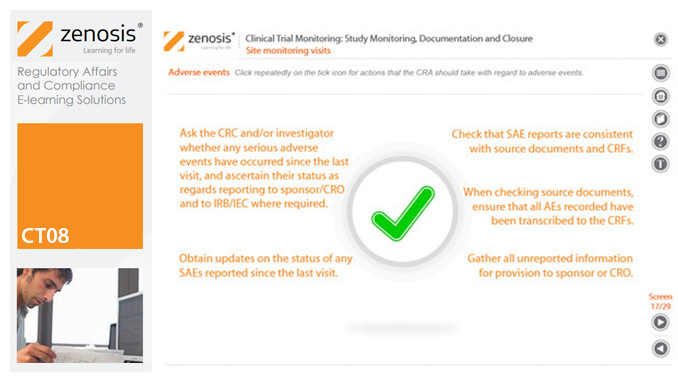
CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
By Zenosis
The sponsor of a clinical trial must arrange for it to be monitored throughout its duration to ensure that the rights and wellbeing of subjects are protected, the trial data are accurate, complete and verified from source documents, and the conduct of the trial complies with the study protocol, Good Clinical Practice and regulatory requirements. In this module we describe how a Clinical Research Associate (CRA) monitors an ongoing trial to its conclusion.
Enhance your medical writing skills with our Level 3 course. From understanding medical journalism to mastering clinical research writing, this CPD Certified program offers comprehensive training for aspiring medical writers and professionals in the healthcare industry.

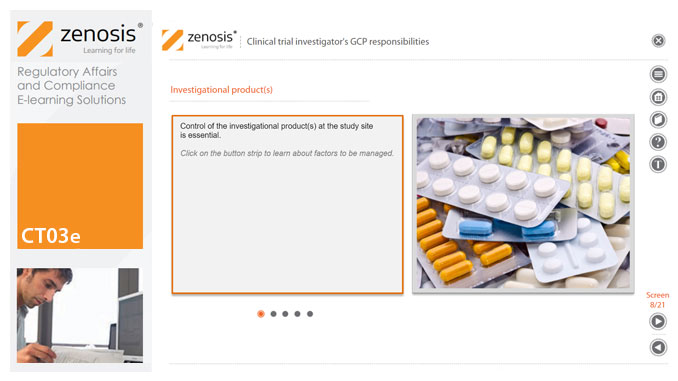
CT03e - Clinical trial investigator’s GCP responsibilities
By Zenosis
A clinical investigator is responsible for conducting the clinical trial in compliance with the study protocol, GCP, medical ethics, and applicable legal requirements. The clinical research community expects that investigators and clinical staff are fully trained in GCP. Duties and functions discussed in this short course include: provision of adequate resources; liaison with IRB/IEC; compliance with protocol; management of investigational product(s), informed consent and data records; and safety reporting.
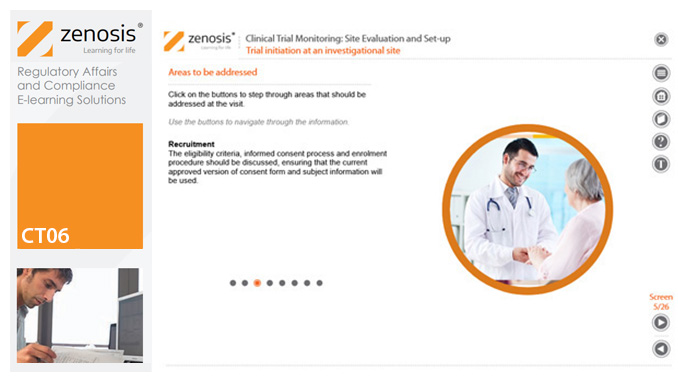
CT06: Clinical Trial Monitoring: Site Evaluation and Setup
By Zenosis
The sponsor of a clinical trial needs to reach agreement with clinical investigators to conduct the trial. The suitability of investigators and their institutional sites, typically hospitals, has to be evaluated, and the trial has to be set up at each site. This module describes the processes involved, focusing particularly on the role of a Clinical Research Associate (CRA) employed or contracted by the sponsor to monitor the trial.
GLP01 - Good Laboratory Practice
By Zenosis
The purpose of GLP is to provide assurance of the quality and reliability of nonclinical study data. GLP covers the planning, performance, monitoring, recording and reporting of studies. Regulatory authorities typically require GLP rules to be followed for nonclinical studies intended to support an application for approval of clinical research or marketing of a product containing the test item. This course outlines the history of GLP and explains why it is important, identifies the penalties that may be incurred for noncompliance, and sets out requirements that need to be met. Learners are also referred to the two main sources of GLP rules: The Organisation for Economic Co-operation and Development’s Principles on Good Laboratory Practice and US Regulation 21 CFR 58: Good Laboratory Practice for Nonclinical Laboratory Studies.

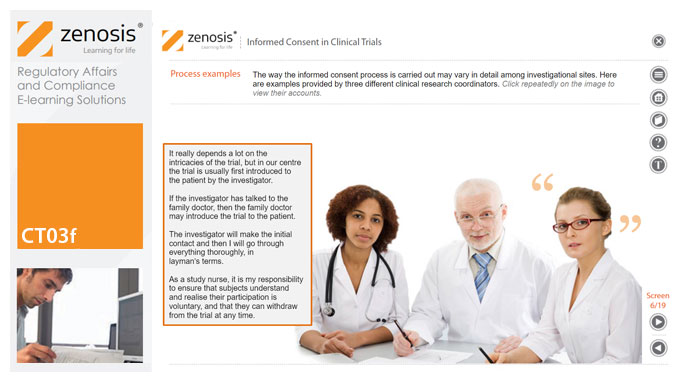
CT03f - Informed consent in clinical trials
By Zenosis
Informed consent in clinical research is an ethical and regulatory requirement. A research subject must enter a study voluntarily, be informed about risks and benefits, and understand the difference between investigation and treatment. Subjects must not be coerced into enrolment, nor must they be enticed by exaggerated claims of benefit. Before they can enrol, all potential subjects must agree, in writing, to participate. In addition to ethical and regulatory imperatives, the potential for litigation by subjects further highlights the importance of rigorous adherence to informed consent principles. In this short course we set out the principles and requirements and provide examples of practical issues confronting healthcare professionals and subjects.
Overview An entry-level medical writer can earn around £35,000, while a more experienced writer can earn around £62,000 per year. If you want to be a part of this lucrative profession, the Medical Writing Training course can be your stepping stone. Our online Medical Writing Training course aims to provide you with quality training in medical writing. The course is divided into 7 modules packed with valuable information. Through these modules, you will learn the core principles of medical writing. Then you will get a clear understanding of how to develop medical reports. The course will also educate you on medical journalism and the process of writing content for medical professionals. At the end of the course, you will receive a recognised certificate of achievement. This certificate will help you prove your proficiency in this area and boost your CV. Enrol today! Course Preview Learning Outcomes Get introduced to the basic principles of medical writing Understand how to conduct reviews and create medical reports Develop your skills in writing pieces for healthcare professionals Learn the process of medical writing in clinical research Explore the vital areas of medical journalism Why Take This Course From John Academy? Affordable, well-structured and high-quality e-learning study materials Meticulously crafted engaging and informative tutorial videos and materials Efficient exam systems for the assessment and instant result Earn UK & internationally recognised accredited qualification Easily access the course content on mobile, tablet, or desktop from anywhere, anytime Excellent career advancement opportunities Get 24/7 student support via email What Skills Will You Learn from This Course? Medical Writing Report Writing Communication Who Should Take this Medical Writing Training Course? Whether you're an existing practitioner or an aspiring professional, this course is an ideal training opportunity. It will elevate your expertise and boost your CV with key skills and a recognised qualification attesting to your knowledge. Are There Any Entry Requirements? This Medical Writing Training course is available to all learners of all academic backgrounds. But learners should be aged 16 or over to undertake the qualification. And a good understanding of the English language, numeracy, and ICT will be helpful. Medical Writing Training Certification After completing and passing the Level 2 Media Studies course successfully, you will be able to obtain a Recognised Certificate of Achievement. Learners can obtain the certificate in hard copy at £14.99 or PDF format at £11.99. Career Pathâ This exclusive Medical Writing Training course will equip you with effective skills and abilities and help you explore career paths such as Medical writer Research Assistant Module 01: Introduction to Medical Writing Introduction to Medical Writing 00:25:00 Module 02: The Career of a Medical Writer The Career of a Medical Writer 00:20:00 Module 03: Medical Writing Essentials Medical Writing Essentials 00:20:00 Module 04: Reviews and Reports Reviews and Reports 00:25:00 Module 05: Medical Journalism and Mass Media Medical journalism and Mass Media 00:25:00 Module 06: Medical Writing for Medical Professionals Medical Writing for Medical Professionals 00:35:00 Module 07: Medical Writing in Clinical Research Medical Writing in Clinical Research 00:30:00 Order Your Certificate and Transcript Order Your Certificates and Transcripts 00:00:00

CT03: ICH Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data. The International Council for Harmonisation’s guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice.
