- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
151 Clinical Practitioner courses
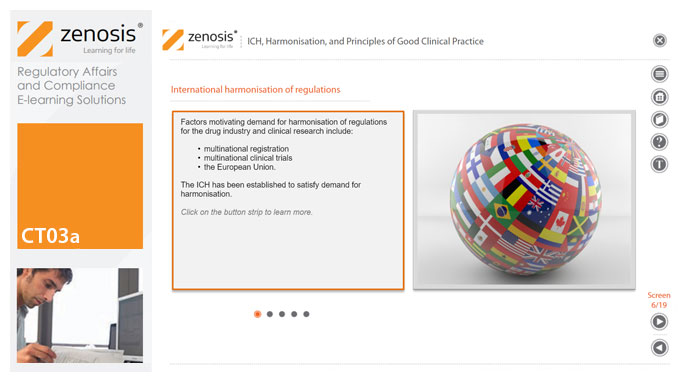
CT03a - ICH, harmonisation, and principles of Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data in applications for marketing approval. The International Council for Harmonisation's guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice. In this short course we describe the ICH’s role in the harmonisation of regulations, introduce its guideline E6, and set out the principles of GCP.
Hypnotic language patterns, for an effective consultation processes, you'll learn rapid transformation, behaviour change and state change techniques. You'll learn how to create sessions for audio, video and group therapy. You'll also learn the rapid phobia release and 3 step rewind. We also start to help you to create you own clinical practise.

Professional Clinical Hypnotherapy Supervision
5.0(23)By The Northern College Of Clinical Hypnotherapy
Supervision is an essential component of professional development in the field of clinical hypnotherapy. It provides a structured space where therapists can reflect on their clinical practice, receive constructive feedback, and explore new strategies to enhance their therapeutic skills. Our supervision sessions are tailored to meet the unique needs of clinical hypnotherapists, ensuring that you receive relevant guidance and support.

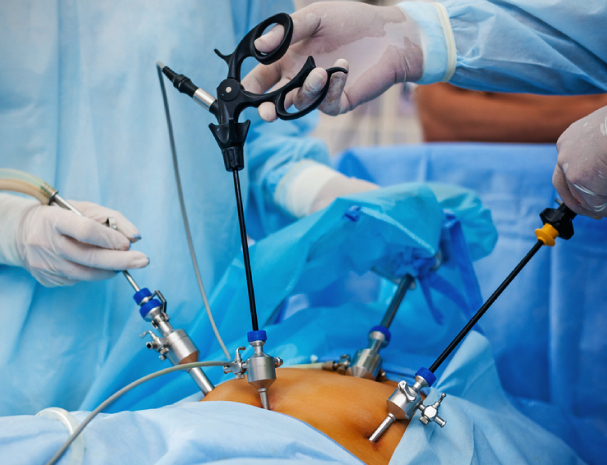
Total Laparoscopic Hysterectomy Course
By CCMIG
Total Laparoscopic Hysterectomy (TLH) Course in London for Gynaecology doctors, trainees and consultants. Theory and hands on training.
Hysteroscopy Course Basic to Advanced
By CCMIG
Hysteroscopy course, hands on training dry and wet-lab and virtual reality simulation.
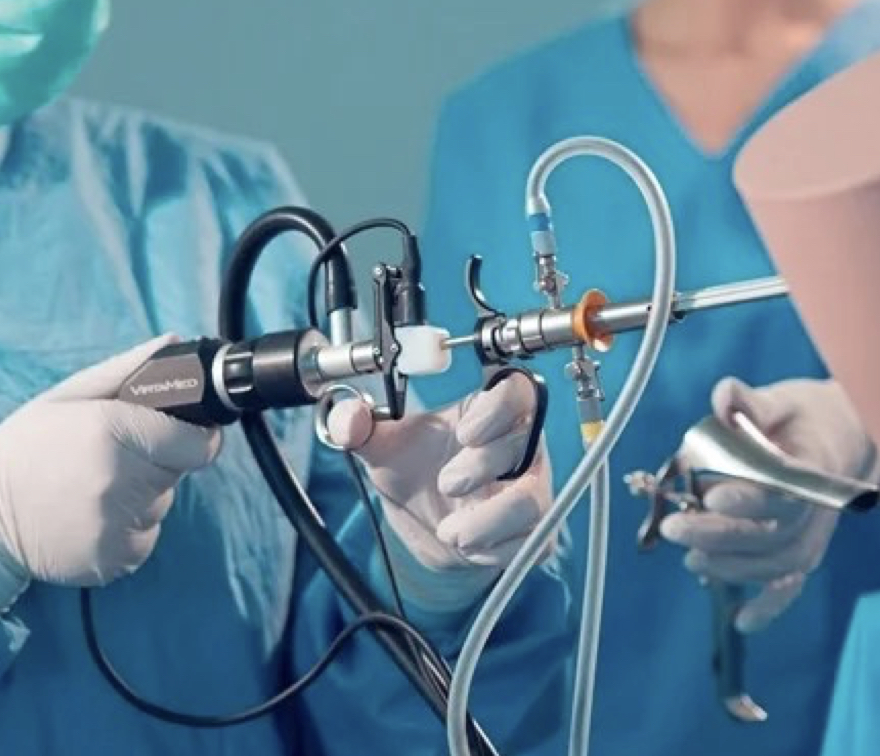
Myomectomy Course (Laparoscopic and Robotic)
By CCMIG
Total Laparoscopic Hysterectomy (TLH) Course in London for Gynaecology doctors, trainees and consultants. Theory and hands on training.

Foundation & Practitioner Diploma in Clinical Hypnotherapy
5.0(26)By The Northern College Of Clinical Hypnotherapy
Our Practitioner Diploma in Clinical Hypnotherapy is a blend of eLearning at your own pace, weekly video conferencing, Masterclasses, and Peer Supervision sessions with interactive and synchronised learning.

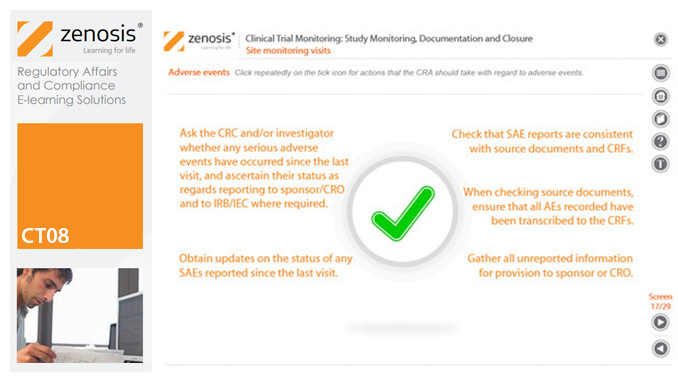
CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
By Zenosis
The sponsor of a clinical trial must arrange for it to be monitored throughout its duration to ensure that the rights and wellbeing of subjects are protected, the trial data are accurate, complete and verified from source documents, and the conduct of the trial complies with the study protocol, Good Clinical Practice and regulatory requirements. In this module we describe how a Clinical Research Associate (CRA) monitors an ongoing trial to its conclusion.
Medical Terminology Training Courses - Level 2
By Mediterm Training
This course leads to the Mediterm Intermediate Award in Medical Terminology (Level 2), studied over approximately 12 weeks (taking more or less time dependent on learner requirements). This course is suitable for those already working in healthcare or those who wish to start a new career in healthcare.
Search By Location
- Clinical Practitioner Courses in London
- Clinical Practitioner Courses in Birmingham
- Clinical Practitioner Courses in Glasgow
- Clinical Practitioner Courses in Liverpool
- Clinical Practitioner Courses in Bristol
- Clinical Practitioner Courses in Manchester
- Clinical Practitioner Courses in Sheffield
- Clinical Practitioner Courses in Leeds
- Clinical Practitioner Courses in Edinburgh
- Clinical Practitioner Courses in Leicester
- Clinical Practitioner Courses in Coventry
- Clinical Practitioner Courses in Bradford
- Clinical Practitioner Courses in Cardiff
- Clinical Practitioner Courses in Belfast
- Clinical Practitioner Courses in Nottingham
