- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
8158 Medicine & Nursing courses
Introduction to Good Manufacturing Practice
By Research Quality Association
Course Information This course offers foundational guidance and practical support tailored for individuals operating within Good Manufacturing Practice (GMP) frameworks. Explore the fundamental prerequisites of a pharmaceutical quality system (PQS) and delve into the application of quality risk management (QRM) principles, aligning with current regulations and guidance. Gain insights into pivotal aspects such as requirements, roles, and responsibilities, encompassing change control, document management, and key documentation essential for effective implementation of GMP with a focus on regulatory inspections and common findings. Is this course for you? Ideal for professionals engaged in GMP across various sectors, including: Research and Development (R&D) Contract Manufacturing Organisations Manufacturing Units Quality Control (QC) Laboratories Auditing Roles. What will you learn? Event objectives - by the end of the course, delegates shall: Have an awareness of the basic requirements of GMP Be aware of UK and EU GMP Rules and Guidance and relevant publications Understand the roles and responsibilities associated with GMP Be able to contribute to and maintain quality documentation Have a basic understanding of product lifecycle and manufacturing Understand the requirements of GMP in the QC laboratory context Have a basic understanding of risk management and mitigation principles Understand the need for quality systems and quality assurance activities Be aware of common regulatory findings. Learning outcomes: delegates will be able to: Implement their role within GMP with confidence and knowledge of the principle requirements Contribute effectively to the GMP quality system and their organisation’s compliance Comprehend where their organisation’s activities sit within the larger GMP arena Know where to seek further information within the published rules and guidance, UK Legislation, European Commission Directives, ICH Guidance and other relevant publications, as well as via the internet. Tutors Tutors will be comprised of (click the photos for biographies): Louise Handy Director, Handy Consulting Ltd Programme Please note timings may be subject to alteration. Day 1 09:30 Introductions and Scope of the Course Understand the group requirements and the tutor's background and experience. 09:45 Background and Regulatory Environment Setting the scene, understanding the context, key legislation. 10:30 Principles of GMP Key points and requirements. 11:15 Break 11:30 Personnel and Responsibilities Management and staff, duties and accountabilities. 12:00 Overview of GMP Manufacturing Basics of the product life cycle. 12:30 Lunch 13:15 Risk Management Workshop Practical exploration of risk and mitigation activities. 14:30 QC Laboratories Activities and practicalities. 15:15 Break 15:30 Compliance Quality Assurance and Self Inspection. 16:15 Question Time A chance for questions on the practicalities of GMP. 16:30 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Learn

Quality Systems for Research Laboratories
By Research Quality Association
Course Information This highly interactive course will provide guidance on why and how to implement a quality system successfully into the research laboratory. By doing so, you will position your innovation for the success it deserves. But leave things as they are and there is a good chance that your science will not realise its full potential should success, and its consequences, come your way. A quality system in your research laboratory is the most effective and efficient way to: Help scientists work more efficiently Ensure discoveries can be defended Protect the value of intellectual property. This course is particularly aimed at those working in early phase research environments which are not constrained by the regulatory requirements of the Good Practice regulations but are producing intellectual property, testing and/or products for the therapeutic market. For organisational reasons, rather than regulatory ones, this is a place where you need to get it right. The programme is delivered by leaders in the field who, quite simply, ‘have done it’. Whether delegates are at senior management level seeking strategic direction, a laboratory head wishing to deliver science that will stand the test of time or a quality professional thrown in at the deep end, this course will provide key insight and practical guidance to underpin future success. Based on risk based systems, tried and tested over many years in the workplace, the programme will help delegates to define, train, implement and monitor the quality of their research, irrespective of field or discipline. Delegates will learn how to help position their organisation for success. Course content: Delegates will be guided thoughtfully through each key component of the process in a stimulating learning environment. The course probes all avenues of the research quality arena, from an initial understanding of the cultural aspects of the scientific discovery environment, to managing quality in outsourced research programmes. Computer systems and e-data security in the research environment will be discussed and pragmatic solutions described to help manage the ballooning cloud of e-data. In addition, the ever blurring boundary between the regulated and non-regulated research environments will be discussed and delegates given perspective on future developments in the field. With this knowledge, delegates will be able to get it ‘right first time’. Is this course for you? The course is designed for all those involved in the research laboratory quality arena and it has been tailored to meet the needs of scientific management, bench scientists and quality professionals alike. Delegates get immediate access to highly experienced tutors who will share their wisdom and insights in an area where few others have been successful. The course is linked with the RQA guidance which builds on years of experience and forms the foundation of the programme. Tutors Tutors will be comprised of (click the photos for biographies): Louise Handy Director, Handy Consulting Ltd Sandrine Bongiovanni Associate Director in Research and Quality Compliance, Novartis Programme Please note timings may be subject to alteration. Day 1 09:00 Registration 09:10 Welcome and Introductions 09:20 History and Overview of the Field Examples of business and regulatory risks and the consequences of low quality in research. A look at the standards and guidelines that exist. 10:00 The Culture, the Politics and the Scientist's Perspective Understanding research environments, the drivers and the challenges. 10:30 Break 10:45 Workshop - Risk Management Thinking about risk management and prioritisation. Looking at the critical factors for the implementations of a successful quality system. 12:15 Workshop - Feedback 12:45 Lunch 13:45 Personnel, Plans, Procedures, Facilities, Equipment, Materials and Reagents Looking at planning the work, defining procedures in a way which promotes robust science without compromising brilliance and ensuring that all these elements are demonstrably fit for their intended purpose. 14:30 Workshop - Assay Validation How much validation is required at what stage? What do we need to validate an assay? 15:00 Workshop - Feedback 15:15 Research, Work Records, Archives and Research Review Data and records which are accurate, attributable, legally attestable and safe to permit reconstruction experiments and studies. Looking at aspects of the work where there is a chance to review, correct or improve the science, the data and the processes. 16:15 Continual Improvement and Quality Systems Reviewing implementation of a quality system, finding opportunities for improvement, understanding culture change. 16:45 Questions and Answers 17:00 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Develop

Good Laboratory Practice Refresher and Hot Topics
By Research Quality Association
Course Information Join us for a comprehensive refresher focusing on crucial Good Laboratory Practice (GLP) requirements, including an emphasis on data integrity, recent developments, and emerging trends gleaned from MHRA inspections. The programme dives into specific domains such as risk assessment, OECD guidance on sponsor influence, and the advisory from OECD on QA. Additionally, delegates can benefit from a dedicated GLP clinic, facilitating discussions on understanding and upholding GLP compliance. Is this course for you? This course is tailored for study directors, principal investigators, test facility management, and QA professionals seeking to refresh their knowledge and responsibilities within the GLP framework. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Registration, Welcome and Introduction 09:20 Development of Good Laboratory Practice A reminder of the history of GLP, its current scope and application, with a synopsis of current UK, European and international standards. 09:50 Roles and Responsibilities of Study Director, Test Facility Management, Principal Investigator, Test Site Management, Study Staff and QA A reminder of the roles and responsibilities with regard to the GLP management and oversight of the Test Facility and the management and control of the study, as defined by GLP. 10:30 Break 10:45 Workshop 1 Workshop 1 Roles and responsibilities 11:15 Influence of Sponsors The published OECD Position Paper No. 21 regarding Possible Influence of Sponsors on conclusions of GLP Studies is reviewed and discussed. 11:45 Data Integrity The fundamentals of data integrity according to the OECD Guidance No. 22 on Data Integrity is discussed along with the responsibilities of Study Director, Test Facility Management, and study staff in ensuring the integrity of the GLP study data. 12:30 Lunch 13:15 Quality Assurance and GLP OECD Advisory No. 23 (Revision of OECD No.4)- A walk through of the changes to the OECD Guidance on the role and activities of Quality Assurance 13:45 Quality Improvement Tools and GLP The tools that might be considered for GLP and their role and operation when used in Test Facilities- OECD Position Paper No.24 published July 2022 14:15 Workshop 2 Workshop 2 Change control 14:30 Risk Assessment How should we assess risk and how can we use the process to assist in evaluation audit findings? 15:00 Break 15:15 Current hot topics in GLP Explore the current issues in Industry and trends /types of Regulatory inspection findings 15:50 GLP Clinic An opportunity to discuss any other issues regarding understanding and maintaining GLP Compliance. 16:30 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Learn

Existential Dialogues: “In Search” with Prof Ernesto Spinelli, Barbara Godoy and International Guest teachers
By Therapy Harley Street
Phenomenological explorations for existential seekers. Ten live dialogues between Prof Ernesto Spinelli and International Existential Therapists which clearly illustrate their journey intertwined with the method of the investigation. We are looking to highlight the role of the researcher as an existential seeker. Workshop – Reflections on Existence through a Phenomenological Method. The two-hour practical module where participants, inspired by the previous presentation and dialogue, explore and elaborate on their practice and research with the guest teacher and Bárbara Godoy. This process ends with the opportunity to experience oneself in a clearing. Here we can reflect on how our research is affecting, disturbing and inspiring our own relationship with Being. TIMES AND DATES: Ten Saturdays 2 pm to 5 pm (UK time) 27 Jan. “Placebo” with Prof Ernesto Spinelli and Bárbara Godoy 24 Feb. “Reflexivity” with Prof Carla Willig and Prof Ernesto Spinelli 23 March “Homecoming” with Prof Robert Romanyshyn and Prof Ernesto Spinelli 27 April “Ungraspable” with Dr Todd Dubose and Prof Ernesto Spinelli 18 May “Homelessness” with Dr Greg Madison and Prof Ernesto Spinelli 22 Jun. “Psychedelics” with Dr Yaqui Martinez and Prof Ernesto Spinelli 20 Jul. “Anxiety” with Prof Kirk Schneider and Prof Ernesto Spinelli 26 Oct. “Intentionality” with Dr Betty Cannon and Prof Ernesto Spinelli 23 Nov. “Vulnerability” with Prof Simon du Plock and Prof Ernesto Spinelli 12 Dec. “Enacting” with Bárbara Godoy and Prof Ernesto Spinelli Full course (including dialogues): £600 (2 pm to 5 pm – UK time) BOOK HERE > Venue: Online Zoom

Level 3 Health and Social Care with Mental Health Nursing
By Kingston Open College
Premium Bundle of all Time | Ofqual Regulation + ATHE Awards + CPD Accreditation | Assessment & Tutor Support Included

Level 3 Health and Social Care with Mental Health Nursing - Regulated Qualification
By Kingston Open College
Premium Bundle of all Time | Ofqual Regulation + ATHE Awards + QLS Endorsement | Assessment & Tutor Support Included

Permanent Makeup Removal & Correction
By Cosmetic College
Course prerequisites This course is available to those with at least one of the following qualifications: We only accept students aged 18 and over. Students must have previous Permanent makeup or microblading training and certification. Must have good written and spoken English Course structure This course includes 4 hours of theory study via our accessible e-learning portal with 5 practical hours. All courses are kept intimate with a maximum of 6 learners to a class. Areas covered within this course are: Clinical setup Infection control PMU Removal Practical training Salt and Saline technique Chemical Removal Colour Correction Professional live demonstrations Pre-study pigment removal and correction
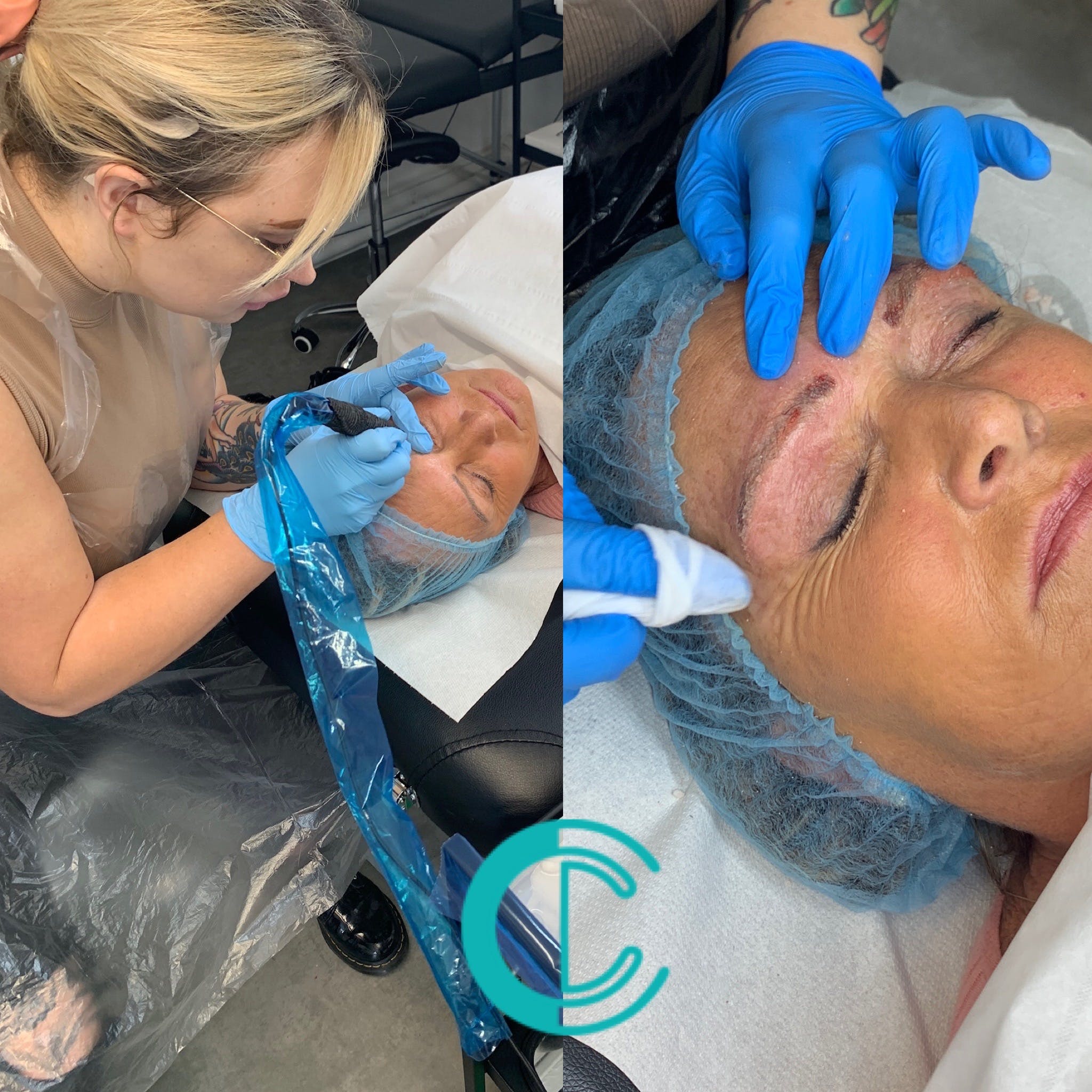
Non Surgical Rhinoplasty Masterclass
By Cosmetic College
Course prerequisites Suitable for both medics and non medics A foundation level dermal filler qualification is required to enrol onto this course. This training course requires you to be an active practising aesthetic injector with a minimum of six months experience with a demonstrable portfolio of client treatments. Course Agenda This intensive course includes 10 hours of theory study via our accessible e-learning portal with 1 practical treatment with models. All courses are kept intimate with a maximum of 4 learners to a class. Areas covered within this course are: Dermal filler injection, cannula techniques and methods for the nose. Danger zones training Start Business up (How to set up a business, insurance, stockist, social media, managed, join our Instagram group) Professional live demonstrations Practical training Areas that are covered in this course: Nose treatments with dermal filler

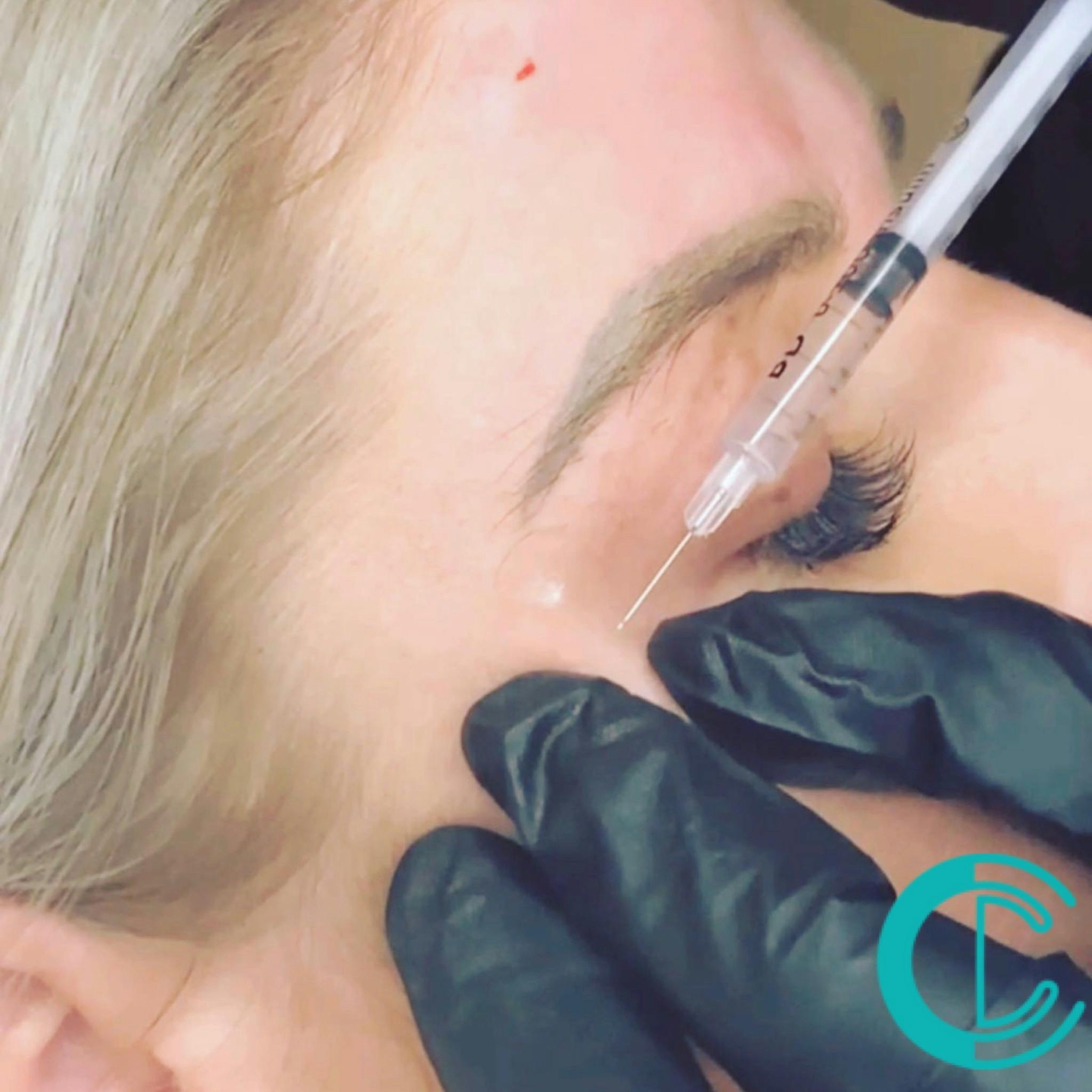
Tear Trough Masterclass
By Cosmetic College
Course prerequisites Suitable for both medics and non medics A foundation level dermal filler qualification is required to enrol onto this course. Course Agenda This intensive course includes 10 hours of theory study via our accessible e-learning portal with 1 practical treatment with models. All courses are kept intimate with a maximum of 4 learners to a class. Areas covered within this course are: Dermal filler injection, cannula techniques and methods for the tear trough. Danger zones training Start Business up (How to set up a business, insurance, stockist, social media, managed, join our Instagram group) Professional live demonstrations Practical training Areas that are covered in this course: Tear Trough, with needle and cannula

Anti Wrinkle Injections Training
By Cosmetic College
Our students gain the product knowledge and practical skills needed to perform foundation anti-wrinkle injections to the upper third of the face. You will learn the fundamentals of facial anatomy, muscle structure, and the ageing process. By understanding these key concepts, you will be able to assess your client's needs accurately and create customised treatment plans to achieve optimal results. Through hands-on practical sessions, you will gain the confidence and proficiency to administer anti-wrinkle injections with precision. Our experienced trainers will guide you through various injection techniques, dosage calculations, and proper injection site selection. Patient safety is our top priority, and you will learn important safety protocols to minimise risks and manage potential complications. By implementing best practices, you will ensure a comfortable and secure experience for your clients. Course Entry Requirements: One or more of the following: Be a medical professional registered to a medical body (NMC, GMC, GDC, GPhC, etc.) Have Level 3 NVQ in Beauty Therapy. Have six months of experience in SPMU, Microblading, and Microneedling) and six months of Anatomy & Physiology Level 3. Have 12 months of experience in advanced beauty treatments (e.g SPMU, Microblading, Microneedling). Course Pre-Study/Practical & Length: Pre Study 1-day on-site training Course Agenda: Product History Face anatomy Injection techniques Ageing process Patient consultation & expectations Tutor demonstration Student practical session Delivery method Areas Glabella lines, Cross feet, Frown lines Course Benefits Student Benefits Enhanced Knowledge and Skills: You will gain in-depth knowledge of facial anatomy, muscle structure, and the ageing process, enabling you to accurately assess clients' needs and develop personalised treatment plans. Through practical training, you will acquire the skills and confidence to administer anti-wrinkle injections with precision. Professional Credibility: Completing this course will establish you as a qualified and competent practitioner in the field of anti-wrinkle injections. Your certification will enhance your professional credibility and increase your chances of securing employment in reputable medical spas, aesthetic clinics, or starting your own practice. Expanded Career Opportunities: The aesthetics industry is continuously growing, and there is a high demand for skilled professionals in the field of anti-wrinkle treatments. By enrolling in this course, you will position yourself for exciting career opportunities and the potential for career advancement. Client Benefits Personalised Treatment Plans: With your enhanced knowledge and skills, you will be able to assess clients' unique facial features, concerns, and expectations. This will enable you to develop customised treatment plans tailored to their specific needs, ensuring optimal results. Safe and Effective Treatments: The comprehensive training you receive will prioritise patient safety and risk management. You will learn proper injection techniques, dosage calculations, and safety protocols, minimising risks and ensuring a safe and comfortable experience for clients. Youthful and Refreshed Appearance: By administering anti-wrinkle injections, you can help clients achieve a smoother, more youthful appearance. By reducing the appearance of wrinkles and fine lines, clients will experience increased confidence and satisfaction with their rejuvenated appearance. Earning Potential By completing this course, you can unlock significant earning potential. As a certified practitioner in anti-wrinkle injections, you can expect competitive salaries and income opportunities in the aesthetics industry. The exact earning potential may vary depending on factors such as your location, experience, and clientele. With the growing demand for anti-wrinkle treatments, you have the opportunity to build a successful career and secure a rewarding salary. Whether you choose to work in established clinics, medical spas, or start your own practice, the ability to offer anti-wrinkle injections can significantly enhance your earning potential. Enrol in our Foundation Anti Wrinkle Injections Training Course today and take the first step towards a lucrative career in aesthetics. Unlock your earning potential and make a positive impact in the lives of your clients. Frequently Asked Questions What topics are covered in the course curriculum? Our course curriculum covers essential topics such as facial anatomy, injection techniques, product selection, client consultation, and post-treatment care. You will receive comprehensive theoretical knowledge and hands-on practical training to ensure a well-rounded learning experience. Do I need to bring my own models for the practical training? No, it is not necessary to bring your own models for the practical training sessions. We provide models for you to practice on under the guidance of our experienced instructors. However, if you prefer to bring your own models, you are welcome to do so. Will I receive a certification upon course completion? Yes, upon successfully completing the course, you will receive a certification that recognises your proficiency in performing anti-wrinkle injections. This certification will enhance your professional credibility and open doors to career opportunities.
Search By Location
- Courses in London
- Courses in Birmingham
- Courses in Glasgow
- Courses in Liverpool
- Courses in Bristol
- Courses in Manchester
- Courses in Sheffield
- Courses in Leeds
- Courses in Edinburgh
- Courses in Leicester
- Courses in Coventry
- Courses in Bradford
- Courses in Cardiff
- Courses in Belfast
- Courses in Nottingham