- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7238 Medicine & Nursing courses delivered On Demand
Level 3, 4 & 5 Pharmacy Assistant and Technician
By Imperial Academy
Level 5 QLS Endorsed Course with FREE Certificate | CPD & CiQ Accredited | 150 CPD Points | Lifetime Access

Level 2, 3 and 5 Pharmacy Dispenser
By Imperial Academy
Level 5 QLS Endorsed Course with FREE Certificate | CPD & CiQ Accredited | 150 CPD Points | Lifetime Access

Level 2, 3 and 5 Pharmacy Assistant - Essential Bundle
By Imperial Academy
Level 5 QLS Endorsed Course with FREE Certificate | CPD & CiQ Accredited |150 CPD Points | Lifetime Access

This Care Training bundle is thoughtfully designed for professionals and learners eager to deepen their knowledge across a broad spectrum of care-related subjects. Covering vital topics from Health and Social Care to specialised areas like Diabetes Awareness, Mental Health Nursing, and End of Life Care Nursing, this bundle offers a valuable collection of courses tailored to support personal and professional development in the care sector. Each module is crafted to provide clear, accessible information that promotes understanding and confidence in delivering quality care. Whether you’re looking to broaden your understanding of medical law, improve awareness of epidemic and pandemic diseases, or develop knowledge in areas such as safe handling of medicines and infection prevention, this course package meets diverse learning needs without the need for physical attendance. Delivered entirely online, it allows you to learn at your own pace, balancing study with other commitments while gaining knowledge essential to today’s health and social care environments. Key Features of Care Training Bundle CPD Accredited Care Training Course Instant PDF certificate Fully online, interactive Care Training course Self-paced learning and laptop, tablet, smartphone-friendly 24/7 Learning Assistance Discounts on bulk purchases Enrol now in this Care Training Bundle course to excel! To become successful in your profession, you must have a specific set of Care Training skills to succeed in today's competitive world. In this in-depth Care Training course, you will develop the most in-demand Care Training skills to kickstart your career, as well as upgrade your existing knowledge & skills. Care Training Curriculum Course 01: Health and Social Care Course 02: Public Health Course 03: Care Planning and Record Keeping Course 04: Diabetes Awareness Course 05: Dyslexia Awareness Course 06: Maternity Care Assistant Course 07: Foster Care Training Course Course 08: End of Life Care Nursing Course 09: Basic Life Support Course 10: Understanding Autism Level 3 Course 11: Drug and Alcohol Awareness Course 12: Psychotherapy and Rehabilitation Counselling Course 13: Mental Health Nursing Course 14: Medical Law Course 15: Safe Handling of Medicines Course 16: Infection Prevention & Control: Health & Safety Consultant Course 17: Epidemic and Pandemic Disease Awareness Course 18: Personal Hygiene Course Course 19: Health and Safety Level 2 Course 20: Basic First Aid Accreditation This Care Training bundle courses are CPD accredited, providing you with up-to-date skills and knowledge and helping you to become more competent and effective in your chosen field. Certification Once you've successfully completed your Care Training course, you will immediately be sent a digital certificate. Also, you can have your printed certificate delivered by post (shipping cost £3.99). CPD 200 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This course is ideal for all employees or anyone who genuinely wishes to learn more about Care Training basics. Requirements No prior degree or experience is required to enrol in this course. Career path This Care Training Course will help you to explore avariety of career paths in the related industry. Certificates Digital certificate Digital certificate - Included Hardcopy Certificate Hard copy certificate - Included Hardcopy Certificate (UK Delivery): For those who wish to have a physical token of their achievement, we offer a high-quality, printed certificate. This hardcopy certificate is also provided free of charge. However, please note that delivery fees apply. If your shipping address is within the United Kingdom, the delivery fee will be only £3.99. Hardcopy Certificate (International Delivery): For all international addresses outside of the United Kingdom, the delivery fee for a hardcopy certificate will be only £10.

PV04: Signal Detection and Management in Pharmacovigilance
By Zenosis
This module provides a guide to signal detection and management for approved products. The subject is presented as a process comprising four stages: signal detection, signal validation, signal analysis and prioritisation, and risk assessment and minimisation.

SUB04: Preparing Submissions in the Common Technical Document (CTD) Format
By Zenosis
The CTD is the internationally recognised standard format for submissions to medicines regulatory authorities. In the European Economic Area, the USA and Canada, the CTD, in its electronic format (eCTD), is mandatory for all applications for marketing approval and all subsequent related submissions. The CTD is accepted in many other countries, being mandatory for new prescription medicines in some. This module explains the rationale for the CTD and provides guidance on its structure and format and the ways in which it is used.

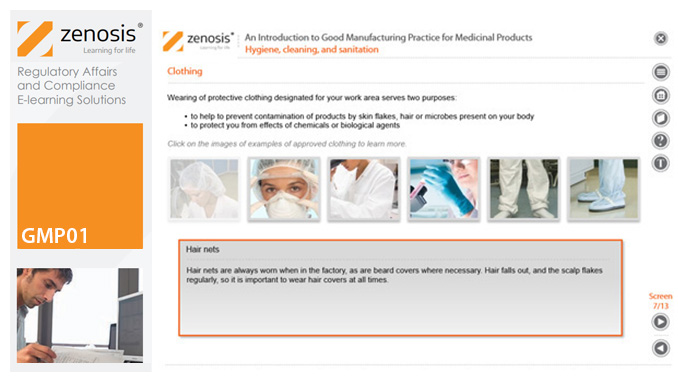
GMP01: An Introduction to Good Manufacturing Practice for Medicinal Products
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. The rules may be written into law or set out in guidance documents from regulatory authorities. Regulators will not allow medicinal products to be placed, or to remain, on the market in their country unless the products can be shown to be manufactured in compliance with GMP. To this end, they carry out inspections of manufacturing plants. Companies that persistently commit serious breaches of GMP requirements have suffered huge fines.
GMP04: Good Manufacturing Practice for the Warehouse
By Zenosis
The warehouse plays a crucial role in a medicinal products factory. This module explains the requirements of Good Manufacturing Practice (GMP) for the warehouse, and how to comply with them.

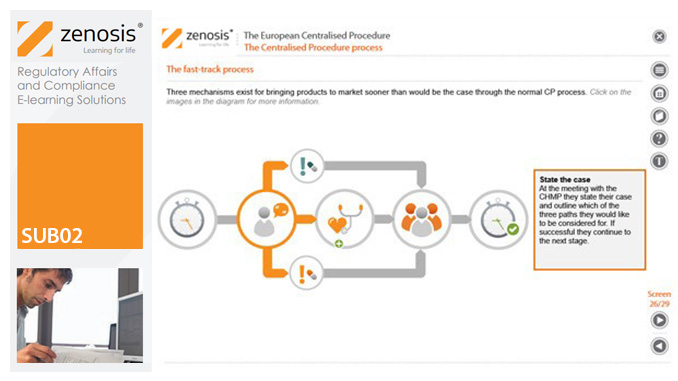
SUB02: The European Centralised Procedure (CP)
By Zenosis
The Centralised Procedure (CP) is one of three routes available to applicants to gain multinational marketing authorisation within the European Economic Area (EEA) on the basis of a single application. In the CP, one successful application leads to a marketing authorisation being issued by the European Commission that applies throughout the EEA. The CP is mandatory for certain types of products.
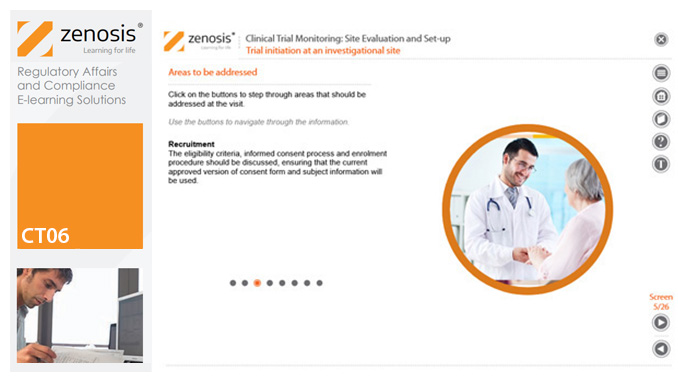
CT06: Clinical Trial Monitoring: Site Evaluation and Setup
By Zenosis
The sponsor of a clinical trial needs to reach agreement with clinical investigators to conduct the trial. The suitability of investigators and their institutional sites, typically hospitals, has to be evaluated, and the trial has to be set up at each site. This module describes the processes involved, focusing particularly on the role of a Clinical Research Associate (CRA) employed or contracted by the sponsor to monitor the trial.